全部照片(2)
About This Item
經驗公式(希爾表示法):
C10H9NO
CAS號碼:
分子量::
159.18
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
97%
mp
81-85 °C (lit.)
官能基
aldehyde
SMILES 字串
Cn1c(C=O)cc2ccccc12
InChI
1S/C10H9NO/c1-11-9(7-12)6-8-4-2-3-5-10(8)11/h2-7H,1H3
InChI 密鑰
IBNGPIOSWCMJGG-UHFFFAOYSA-N
應用
1-Methylindole-2-carboxaldehyde may be used in the synthesis of indole-based melatonin analog hydrazone derivatives and (E)-5-((benzyloxy)methyl)-5-(((tert-butyldiphenylsilyl)oxy)methyl)-3-((1-methyl-1H-indol-2-yl)methylene)dihydrofuran-2(3H)-one.
Reactant for preparation of:
- Deazapurine isosteres from acylindoles via pyridine ring annulation
- 2,4-dichlorocinnamohydroxamic acid analogs for enhancing pharmacokinetics of botulinum neurotoxin serotype A protease inhibitors
- Tetrahydrocarbazoles via organocatalytic cascade Friedel-Crafts alkylation/Michael addition/aromatization reaction
- Azides, imines and amines via flow processes of amines and trimethylsilyl azide and aza-Wittig reaction
- 3-indolylpyridinedicarbonitriles as anti-inflammatory agents
- Bis(indolyl)methanes as antimicrobial and antioxidant agents
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Sibel Suzen et al.
Journal of enzyme inhibition and medicinal chemistry, 28(6), 1143-1155 (2012-09-22)
Melatonin (MLT) is a strong free-radical scavenger, which protects the body from the effects of oxidants. In recent years, MLT have been described resulting in much attention in the development of synthetic compounds possessing. As a part of our ongoing
Noga Gal et al.
Chembiochem : a European journal of chemical biology, 12(15), 2331-2340 (2012-10-30)
N-methyl-substituted diacylglycerol-indololactones (DAG-indololactones) are newly synthesized effectors of protein kinase C (PKC) isoforms and exhibit substantial selectivity between RasGRP3 and PKCα. We present a comprehensive analysis of membrane interactions and biological activities of several DAG-indololactones. Translocation and binding activity assays
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務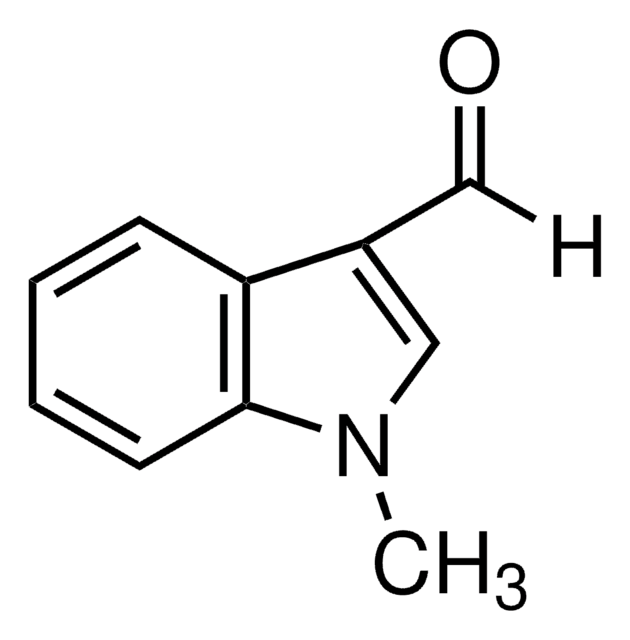

![苯并[b]噻吩-2-甲醛 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)