推薦產品
品質等級
化驗
≥99.0% (HPLC)
反應適用性
reaction type: Coupling Reactions
mp
140 °C (dec.) (lit.)
應用
peptide synthesis
儲存溫度
2-8°C
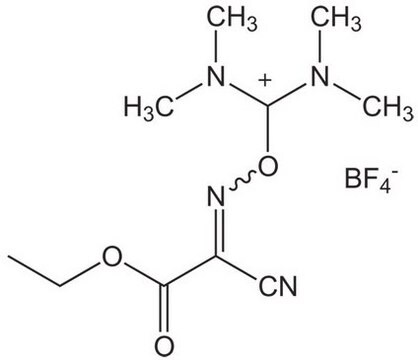
SMILES 字串
F[B-](F)(F)F.CN(C)C(\ON1C=CC=CC1=O)=[N+](/C)C
InChI
1S/C10H16N3O2.BF4/c1-11(2)10(12(3)4)15-13-8-6-5-7-9(13)14;2-1(3,4)5/h5-8H,1-4H3;/q+1;-1
InChI 密鑰
CZQGINAUZYECAI-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
Reagent for:
Amidation
Peptide Coupling
Esterification of nucleosides to solid phase supports for oligonucleoside synthesis
Amidation
Peptide Coupling
Esterification of nucleosides to solid phase supports for oligonucleoside synthesis
其他說明
多肽合成的偶联剂,特别适合于无外消旋作用的片段缩合反应
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Sens. 1A
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
R T Pon et al.
Bioconjugate chemistry, 10(6), 1051-1057 (1999-11-24)
Nucleosides can be esterified to solid-phase supports using uronium or phosphonium coupling reagents and a coupling additive, such as 1-hydroxybenzotriazole (HOBT), 7-aza-1-hydroxybenzotriazole (HOAT), N-methylimidazole (NMI), or 4-(dimethylamino)pyridine (DMAP). However, DMAP was far superior to other additives and high nucleoside loadings
C M Huntley et al.
Nucleosides, nucleotides & nucleic acids, 20(4-7), 731-733 (2001-09-21)
The synthesis of 1-(beta-D-ribofuranosyl)pyridin-2-one-3-carboxylic acid and the 3-carboxamide as well as a short series of 3N-carboxamides, prepared by TPTU/HOBt coupling of primary amines with 1-(beta-D-ribofuranosyl)pyridin-2-one-3-carboxylic acid, and their evaluation as anti-infective agents is described.
Tomohisa Sawada et al.
Journal of the American Chemical Society, 133(19), 7336-7339 (2011-04-28)
Artificial mimicry of α-helices offers a basis for development of protein-protein interaction antagonists. Here we report a new type of unnatural peptidic backbone, containing α-, β-, and γ-amino acid residues in an αγααβα repeat pattern, for this purpose. This unnatural
Knorr, R, et al.
Tetrahedron Letters, 30, 1927-1927 (1989)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務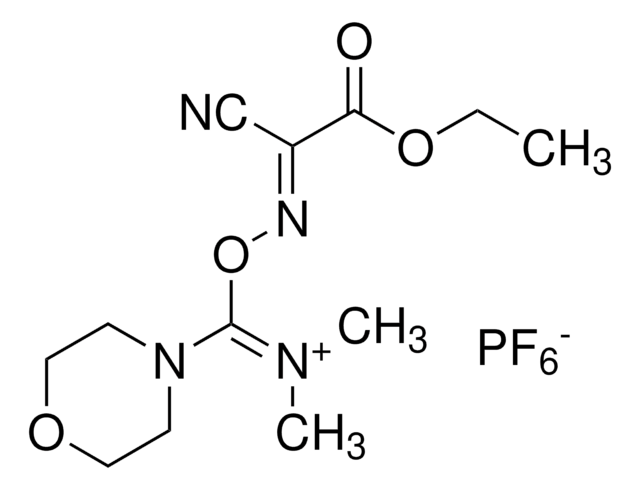




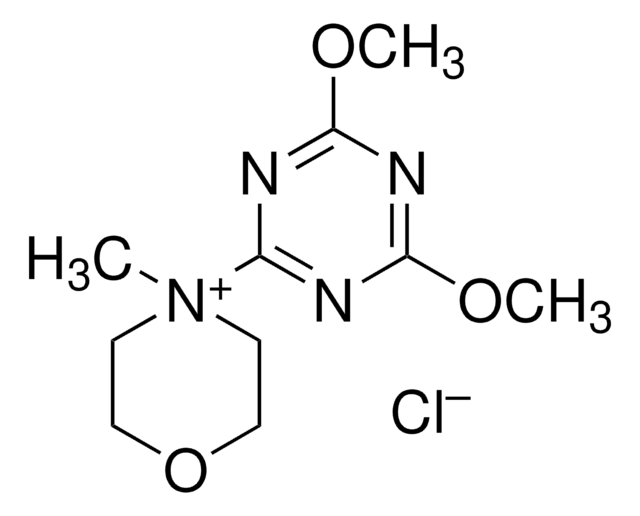
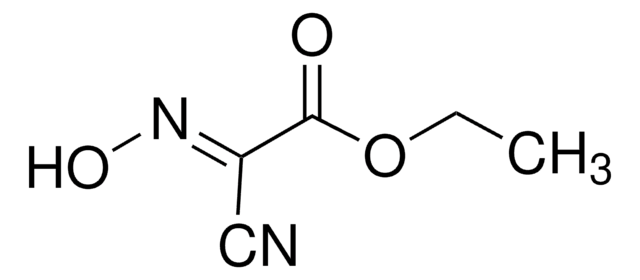
![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)