375217
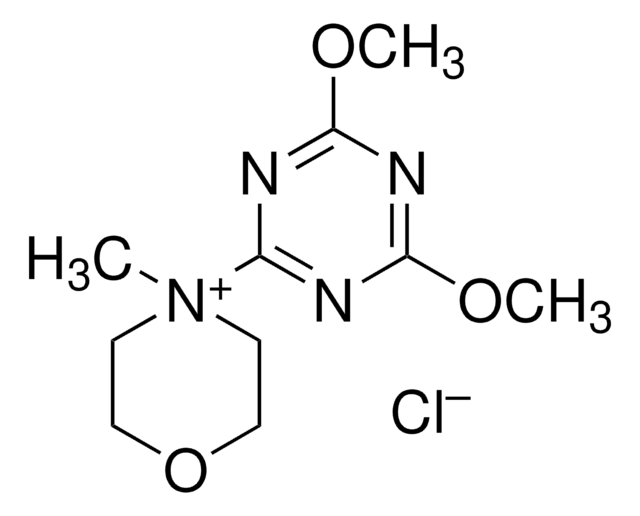
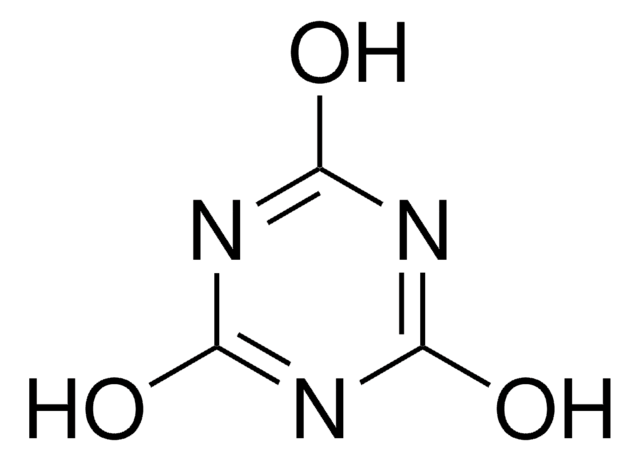
2-氯-4,6-二甲氧基-1,3,5-三嗪
97%
同義詞:
2,4-二甲氧基-6-氯-1,3,5-三嗪, 2,4-二甲氧基-6-氯-s-三嗪, 2-氯-4,6-二甲氧基-1,3,5-三嗪, 2-氯-4,6-二甲氧基-s-三嗪, 4,6-二甲氧基-2-氯-s-三嗪, 6-氯-2,4-二甲氧基-s-三嗪, CDMT, 氯二甲氧基三嗪
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C5H6ClN3O2
CAS號碼:
分子量::
175.57
Beilstein:
148988
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
品質等級
化驗
97%
形狀
solid
mp
71-74 °C (lit.)
官能基
chloro
SMILES 字串
COc1nc(Cl)nc(OC)n1
InChI
1S/C5H6ClN3O2/c1-10-4-7-3(6)8-5(9-4)11-2/h1-2H3
InChI 密鑰
GPIQOFWTZXXOOV-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
2-氯-4,6-二甲氧基-1,3,5-三嗪是一种稳定但高度活性的肽偶联剂。它被报道为多肽纯化的有效偶联试剂。已报道 2-氯-4,6-二甲氧基-1,3,5-三嗪的催化酰胺化反应。已报道制备多千克 2-氯-4,6-二甲氧基-1,3,5-三嗪的方法。
應用
2-氯-4,6-二甲氧基-1,3,5-三嗪可用于以下研究:
- 亲和标记的模块化方法 (MoAL) 对链霉亲和素的特异性标记。
- 通过与 2-羟基-4,6-二甲氧基-1,3,5-三嗪-2-基)醚反应制备双(4,6-二甲氧基-1,3,5-三嗪-2-基)醚。
- 通过与多种叔胺反应制备 2-(4,6-二甲氧基-1,3,5-三嗪基)三烷基铵盐。
- 在四氢呋喃中与 N -甲基吗啉偶联合成 4-(4,6-二甲氧基-1,3,5-三嗪-2-基)-4-甲基吗啉氯化物。
一种稳定并且具高反应性的肽偶联剂。
訊號詞
Danger
危險聲明
危險分類
Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Munetaka Kunishima et al.
Chemical & pharmaceutical bulletin, 61(8), 882-886 (2013-08-02)
Effect of the basic property of reactants (tertiary amine catalysts, a substrate amine, and acid neutralizers) on catalytic dehydrocondensation between a carboxylic acid and an amine by using 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) was studied. The reaction yield was affected by the acid-base
An improved procedure for the large scale preparation of 2-chloro-4, 6-dimethoxy-1, 3, 5-triazine.
Cronin JS, et al.
Synthetic Communications, 26(!8), 3491-3494 (1996)
2-Chloro-4, 6-dimethoxy-1, 3, 5-triazine. A new coupling reagent for peptide synthesis.
Kaminski ZJ.
Synthesis, 10, 917-920 (1987)
Konrad Jastrzabek et al.
Chemistry & biodiversity, 10(5), 952-961 (2013-05-18)
Bis(4,6-dimethoxy-1,3,5-triazin-2-yl) ether (4) was prepared by treatment of 2-hydroxy-4,6-dimethoxy-1,3,5-triazine with 2-chloro-4,6-dimethoxy-1,3,5-triazine in 61% yield. Ether 4, isoelectronic with pyrocarbonates, was found capable to activate carboxylic acids in the presence of 1,4-diazabicyclo[2.2.2]octane (DABCO) to yield, under mild reaction conditions, superactive triazine
Munetaka Kunishima et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(49), 15856-15867 (2012-10-13)
The reaction of 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) with various nitrogen-containing compounds, particularly tertiary amines (tert-amines), has been studied for the preparation of 2-(4,6-dimethoxy-1,3,5-triazinyl)trialkylammonium salts [DMT-Am(s)]. DMT-Ams derived from aliphatic tert-amines exhibited activity for the dehydrocondensation between a carboxylic acid and an amine
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務