推荐产品
品質等級
化驗
≥95% (HPLC)
形狀
oil
顏色
colorless to light yellow
儲存溫度
2-8°C
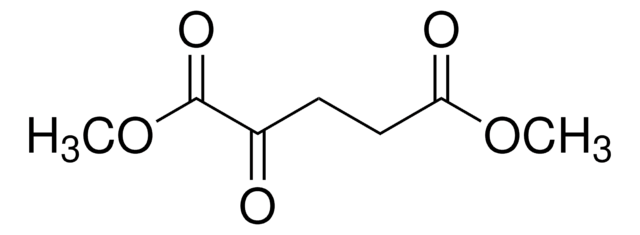
SMILES 字串
O=C(C(CCC(OCC)=O)=O)OCC1=CC=CC(C(F)(F)F)=C1
InChI
1S/C15H15F3O5/c1-2-22-13(20)7-6-12(19)14(21)23-9-10-4-3-5-11(8-10)15(16,17)18/h3-5,8H,2,6-7,9H2,1H3
InChI 密鑰
OKDCLZOKKGLMIX-UHFFFAOYSA-N
生化/生理作用
A cell-permeable, bioavailable & aqueous stable α-ketoglutarate prodrug that selectively induces PHD-dependent cell death in hypoxic cells
ETaKG is a cell-permeable, aqueous stable, bioavailable, non-toxic α-ketoglutarate/2-oxoglutarate (α-KG/2-OG) diester derivative that readily undergoes hydrolysis by cytosolic esterases to α-KG. The monoester analog, TaKG is also membrane permeant. ETaKG elevates intracellular [α-KG] by ~15-fold in a prologned fashion, destabilizes HIF-1α/2α and reactivates prolyl hydroxylases (PHDs) in hypoxic HCT116, A431 and RPE cells at 2 mM. Further, shown to selectively trigger PHD-dependent cell death in hypoxic cells and decreases cellular [ATP] and glucose uptake. ETaKG has shown to reduce glucose metabolism, elevate [α-KG] levels and suppress A375 xenograft tumor growth (750 mg/kg of mice, p.o., q.d., 14 days). Also, induces mTORC1 lysosomal translocation and stimulates mTORC1 activity in the absence of glutaminolysis in U2OS cells.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门