推荐产品
product name
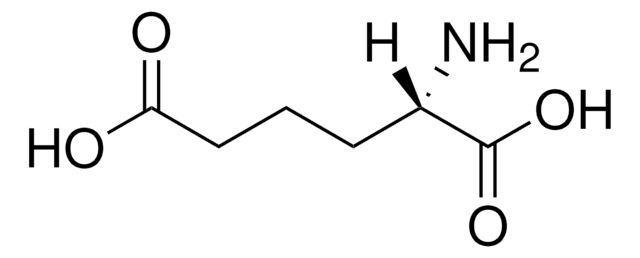
5-羟基-DL-赖氨酸 盐酸盐,
化驗
≥98% (TLC)
品質等級
形狀
powder
顏色
white
mp
225 °C (dec.) (lit.)
應用
detection
peptide synthesis
SMILES 字串
Cl.NCC(O)CCC(N)C(O)=O
InChI
1S/C6H14N2O3.ClH/c7-3-4(9)1-2-5(8)6(10)11;/h4-5,9H,1-3,7-8H2,(H,10,11);1H
InChI 密鑰
MJXVOTKVFFAZQJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
- Characterization of acetyl-CoA: L-lysine N6-acetyltransferase, which catalyses the first step of carbon catabolism from lysine in Saccharomyces cerevisiae.: This research investigates the enzyme acetyl-CoA: L-lysine N6-acetyltransferase, which initiates the catabolism of lysine in Saccharomyces cerevisiae. Utilizing DL-5-Hydroxylysine hydrochloride, the study provides insights into the metabolic pathways and regulatory mechanisms of lysine degradation, contributing to the broader understanding of amino acid metabolism in yeast (Bode et al., 1993).
生化/生理作用
DL-5-羟基赖氨酸是5-羟基赖氨酸的D-和L-对映异构体的外消旋混合物,可用作自由基诱导的蛋白质氧化的潜在靶标。
其他說明
混合DL和DL-allo
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
R Bode et al.
Archives of microbiology, 160(5), 397-400 (1993-01-01)
The carbon catabolism of L-lysine starts in Saccharomyces cerevisiae with acetylation by an acetyl-CoA:L-lysine N6-acetyltransferase. The enzyme is strongly induced in cells grown on L-lysine as sole carbon source and has been purified about 530-fold. Its activity was specific for
B Morin et al.
Chemical research in toxicology, 11(11), 1265-1273 (1998-11-17)
gamma-Irradiation of several amino acids (Val, Leu, Ile, Lys, Pro, and Glu) in the presence of O2 generates hydroperoxides. We have previously isolated and characterized valine and leucine hydroperoxides, and hydroxides, and have detected these products in both isolated systems
M Droux et al.
Archives of biochemistry and biophysics, 316(1), 585-595 (1995-01-10)
Cystathionine beta-lyase, the second enzyme of the transsulfuration pathway leading to homocysteine synthesis was purified over 16,000-fold from spinach (Spinacia oleracea L.) leaf chloroplasts (soluble fraction). Enzyme activity was followed along the purification scheme by either a colorimetric method for
N Zhang et al.
International journal of plant sciences, 160(3), 511-519 (2001-09-07)
Lactuca sativa cv. Baijianye seedlings do not normally produce lateral roots, but removal of the root tip or application of auxin, especially indole-butyric acid, triggered the formation of lateral roots. Primordia initiated within 9 h and were fully developed after
Lasanthi P Jayathilaka et al.
Organic letters, 6(21), 3659-3662 (2004-10-08)
[reaction: see text] L-alpha-(1-Cyclobutenyl)glycine (1-Cbg) was targeted as a potentially translatable analogue of isoleucine and valine and as a useful building block for peptides. An enantioselective synthesis was executed in which the key step was diastereoselective addition of 1-cyclobutenylmagnesium bromide
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门