推荐产品
产品名称
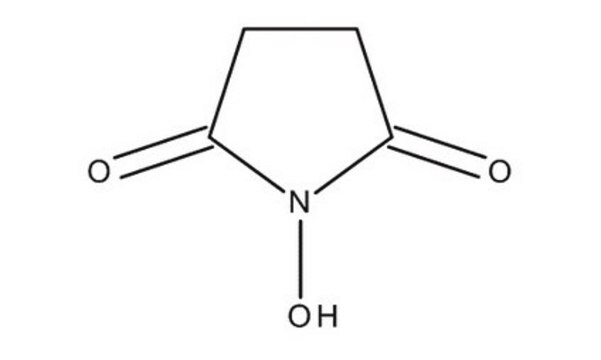
N-羟基丁二酰亚胺, purum, ≥97.0% (T)
等級
purum
品質等級
化驗
≥97.0% (T)
形狀
solid
反應適用性
reaction type: Addition Reactions
mp
95-98 °C (lit.)
95-98 °C
應用
peptide synthesis
官能基
imide
SMILES 字串
ON1C(=O)CCC1=O
InChI
1S/C4H5NO3/c6-3-1-2-4(7)5(3)8/h8H,1-2H2
InChI 密鑰
NQTADLQHYWFPDB-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
- 合成N-琥珀星氩氨-3(2-吡啶二硫代)-酸酯,这是一种异双功能试剂,用于蛋白和蛋白偶联以及脂肪族硫醇插入蛋白。
- 合成长链脂肪酸的NHS酯
- NHS可以活化固定在钛表面的膦酸单层,用于结合蛋白。
其他說明
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
商品
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门