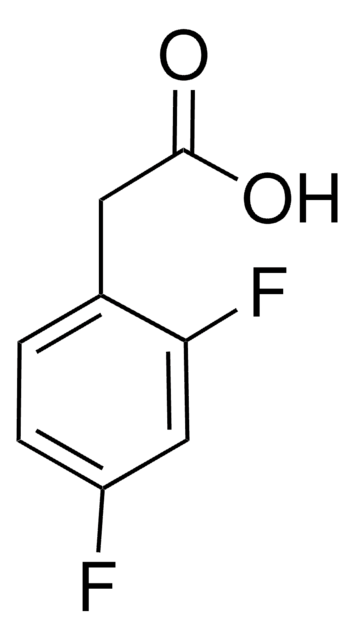
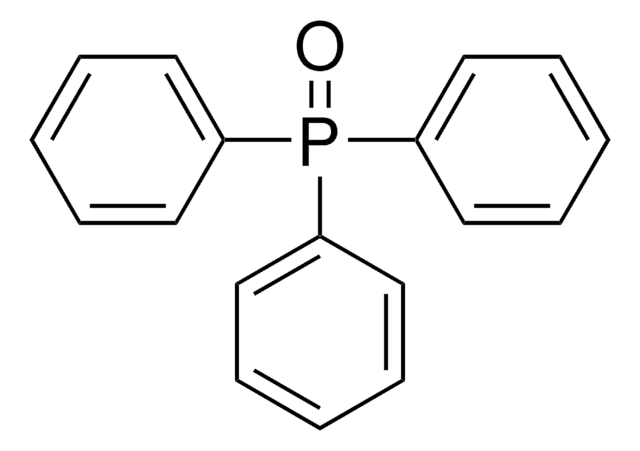
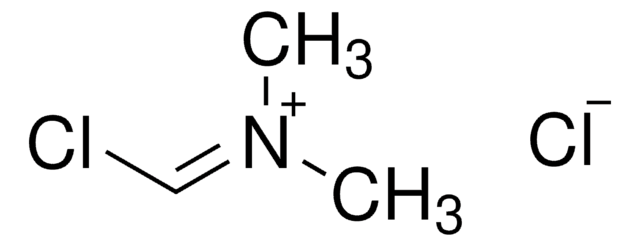
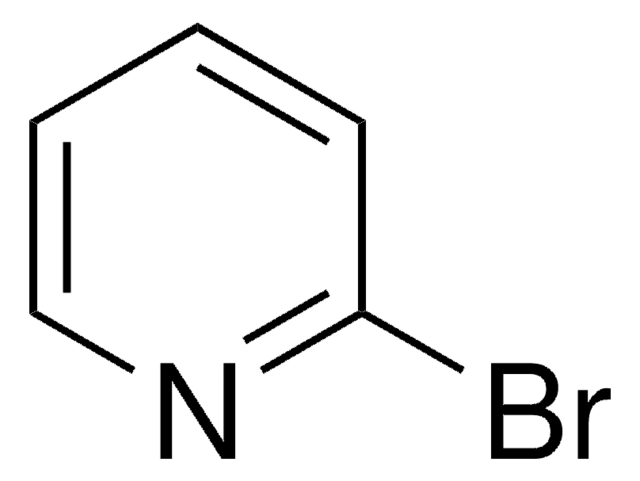
推荐产品
形狀
liquid
反應適用性
reagent type: oxidant
濃度
2.0 M in methylene chloride
密度
1.335 g/mL at 25 °C
官能基
acyl chloride
SMILES 字串
ClC(=O)C(Cl)=O
InChI
1S/C2Cl2O2/c3-1(5)2(4)6
InChI 密鑰
CTSLXHKWHWQRSH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
草酰氯一般用作二甲基亚砜活化剂 和氯化剂,将羧酸转化为酸性氯化物。
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
標靶器官
Central nervous system
儲存類別代碼
4.3 - Hazardous materials which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Synthesis and characterization of highly soluble and heat stable new poly (amide-ether) s containing pyridine rings in the main chain.
Banihashemi A and Vakili MR
e-Polymers, 8(1) (2008)
Catalytic syntheses of N-heterocyclic ynones and ynediones by in situ activation of carboxylic acids with oxalyl chloride.
Christina Boersch et al.
Angewandte Chemie (International ed. in English), 50(44), 10448-10452 (2011-09-13)
Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide" activated" by oxalyl chloride.
Mancuso AJ, et al.
The Journal of Organic Chemistry, 43(12), 2480-2482 (1978)
Benoît Heurtaux et al.
The Journal of organic chemistry, 70(4), 1474-1477 (2005-02-12)
[reaction: see text] Several natural pulvinic acids were synthesized. Silyl ketene acetals derived from methyl arylacetates (4 equiv) reacted with oxalyl chloride at -78 degrees C, without the need of adding a catalyst. After treatment of the crude diketones with
Peter J Manley et al.
Organic letters, 4(18), 3127-3129 (2002-08-31)
[reaction: see text] A mild, practical, one-pot method for the generation of imidoyl chlorides and their subsequent in situ reaction with pyridine-1-oxides is described. The imidoyl chlorides were formed from the reaction of secondary amides with a stoichiometric amount of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持