P7768
Pyruvate Kinase from rabbit muscle
Type VII, buffered aqueous glycerol solution, 350-600 units/mg protein
Synonym(s):
ATP:pyruvate 2-O-phosphotransferase, PK
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
eCl@ss:
32160410
NACRES:
NA.54
Recommended Products
type
Type VII
Quality Level
form
buffered aqueous glycerol solution
specific activity
350-600 units/mg protein
mol wt
237 kDa
concentration
2.0-20.0 mg/mL
foreign activity
lactic dehydrogenase and creatine phosphokinase ≤0.01%
phosphoglucomutase and myokinase ≤0.05%
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
Research Area: Cell Signaling
Pyruvate kinase plays a role in regulating cell metabolism. There are four pyruvate kinase isoforms in mammals (PKM1, PKM2, PKR, PKL). Mammalian pyruvate kinase is a tetrameric protein composed of identical subunits, arranged in a dimer-of-dimers configuration. Each monomer contains one active site and consists of three main domains- designated A, B, and C-along with a small N-terminal domain. The M2 isoform of pyruvate kinase (PKM2) supports anabolic metabolism and is expressed in cancer and normal tissue.
Pyruvate kinase plays a role in regulating cell metabolism. There are four pyruvate kinase isoforms in mammals (PKM1, PKM2, PKR, PKL). Mammalian pyruvate kinase is a tetrameric protein composed of identical subunits, arranged in a dimer-of-dimers configuration. Each monomer contains one active site and consists of three main domains- designated A, B, and C-along with a small N-terminal domain. The M2 isoform of pyruvate kinase (PKM2) supports anabolic metabolism and is expressed in cancer and normal tissue.
Application
Pyruvate Kinase from rabbit muscle has been used as a component in refolding buffer for malate dehydrogenase (MDH) assay. It has also been used as a supplement in assay buffer for phosphoglycerate mutases (PGM) enzyme assays.
Pyruvate kinase from Sigma has been used in the preparation of cell free extract from BL21 CP strain of E. coli culture for highly productive cell-free protein expression. The enzyme has been used to assay the pyruvate kinase activity by incubating with eluted DAPk (Death-associated protein kinase), a serine/threonine kinase that binds and activates pyruvate kinase.
Biochem/physiol Actions
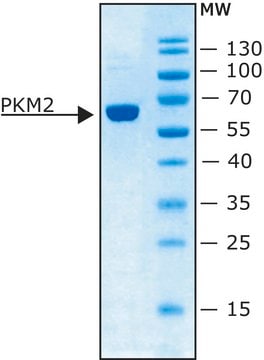
Molecular Weight: 237 kDa and exists as a tetramer of four equal subunits of molecular weight 57 kDa.
Isoelectric Point: 7.6
Optimal pH: ∼7.5
Optimal Temperature: 25°C
ΕA280 = 0.54 for 1 mg(p)/ml, 1 cm path
Reported KM values are ATP (0.86 mM), pyruvate (10 mM), ADP (0.3 mM), and PEP (0.07 mM) in Tris buffer at pH 7.4 and 30 °C. Pyruvate kinase is highly specific for phosphoenolpyruvate, but can utilize other dinucleotide triphosphates as substrates in place of ATP including GTP, ITP, dATP, UTP, and CTP.
Isoelectric Point: 7.6
Optimal pH: ∼7.5
Optimal Temperature: 25°C
ΕA280 = 0.54 for 1 mg(p)/ml, 1 cm path
Reported KM values are ATP (0.86 mM), pyruvate (10 mM), ADP (0.3 mM), and PEP (0.07 mM) in Tris buffer at pH 7.4 and 30 °C. Pyruvate kinase is highly specific for phosphoenolpyruvate, but can utilize other dinucleotide triphosphates as substrates in place of ATP including GTP, ITP, dATP, UTP, and CTP.
Pyruvate kinase catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to ADP. This transfer yields one molecule of pyruvate and one molecule of ATP. Molecular Weight: 237 kDa and exists as a tetramer of four equal subunits of molecular weight 57 kDa.
Isoelectric Point: 7.6
Optimal pH: ∼7.5
Isoelectric Point: 7.6
Optimal pH: ∼7.5
Pyruvate kinase is an enzyme involved in glycolysis and gluconeogenesis. Product P7768 is from rabbit muscle and is provided in a buffered aqueous glycerol solution and has been used in PGM enzyme assays to determine phosphoglycerate mutase activity. Pyruvate kinase is also used to study pyruvate kinase (PK) deficiency
Unit Definition
One unit will convert 1.0 μmole of phospho(enol)pyruvate to pyruvate per min at pH 7.6 at 37 °C.
Physical form
Solution in 50% glycerol containing 0.01 M phosphate, pH 7.0
Analysis Note
Protein determined by biuret.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yinhua Zhang et al.
The Journal of biological chemistry, 279(35), 37185-37190 (2004-07-06)
Phosphoglycerate mutases catalyze the interconversion of 2- and 3-phosphoglycerate in the glycolytic and gluconeogenic pathways. They exist in two unrelated forms that are either cofactor (2,3-diphosphoglycerate)-dependent or cofactor-independent. The two enzymes have no similarity in amino acid sequence, tertiary structure
S M Ayala Mariscal et al.
Nature communications, 13(1), 4692-4692 (2022-08-11)
Huntington's disease is a neurodegenerative disease caused by an expanded polyQ stretch within Huntingtin (HTT) that renders the protein aggregation-prone, ultimately resulting in the formation of amyloid fibrils. A trimeric chaperone complex composed of Hsc70, DNAJB1 and Apg2 can suppress
Studies on pyruvate kinase (PK) deficiency. II. Electrophoretic, kinetic and immunological studies on pyruvate kinase of erythrocytes and other tissues.
K Imamura et al.
Journal of biochemistry, 74(6), 1165-1175 (1973-12-01)
Hager Souabni et al.
Communications biology, 4(1), 493-493 (2021-04-24)
Tripartite efflux pumps built around ATP-binding cassette (ABC) transporters are membrane protein machineries that perform vectorial export of a large variety of drugs and virulence factors from Gram negative bacteria, using ATP-hydrolysis as energy source. Determining the number of ATP
I Mor et al.
Oncogene, 31(6), 683-693 (2011-07-05)
Death-associated protein kinase (DAPk), a multi-domain serine/threonine kinase, regulates numerous cell death mechanisms and harbors tumor suppressor functions. In this study, we report that DAPk directly binds and functionally activates pyruvate kinase M2 (PKM2), a key glycolytic enzyme, which contributes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service