F6008
Ficin from fig tree latex
lyophilized powder
Synonym(s):
Debricin, Ficain, higueroxyl delabarre
About This Item
Recommended Products
biological source
fig tree (latex)
Quality Level
form
lyophilized powder
specific activity
≥1.0 units/mg protein
mol wt
23.8 kDa
composition
Protein, 80-100% biuret
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
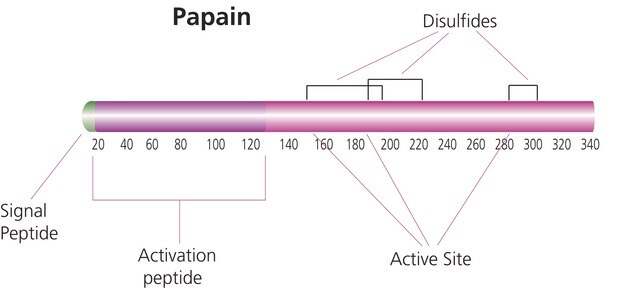
pI: 9.0
Application
- in the proteolysis of tendon collagen
- in the digestion of Chlorophyta Ulva rigida
- to investigate it effect on protease-activated receptors 2 and 4 activation to induce itch in skin
Biochem/physiol Actions
Unit Definition
Physical form
Preparation Note
inhibitor
substrate
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
This procedure may be used for all Ficin products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service