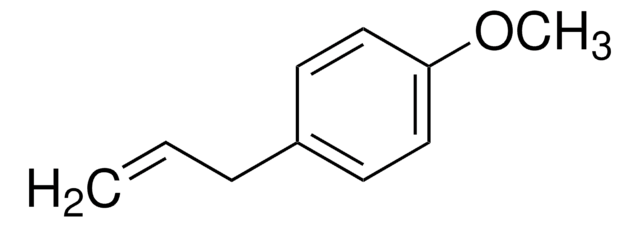
51782
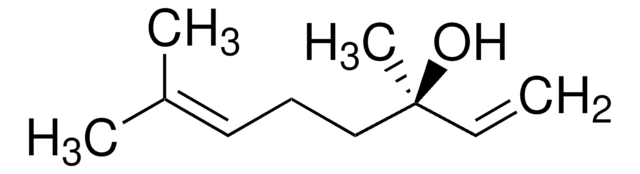
Linalool
analytical standard
Synonym(s):
(±)-3,7-Dimethyl-1,6-octadien-3-ol, (±)-3,7-Dimethyl-3-hydroxy-1,6-octadiene
About This Item
Recommended Products
grade
analytical standard
Quality Level
vapor pressure
0.17 mmHg ( 25 °C)
assay
≥99.0% (GC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.462 (lit.)
bp
194-197 °C/720 mmHg (lit.)
density
0.87 g/mL at 25 °C (lit.)
application(s)
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
format
neat
storage temp.
2-8°C
SMILES string
C\C(C)=C\CCC(C)(O)C=C
InChI
1S/C10H18O/c1-5-10(4,11)8-6-7-9(2)3/h5,7,11H,1,6,8H2,2-4H3
InChI key
CDOSHBSSFJOMGT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
171.0 °F - Pensky-Martens closed cup
flash_point_c
77.2 °C - Pensky-Martens closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
Protocols
-(+)-Limonene, purum, ≥98.0% (sum of enantiomers, GC); Geranyl tiglate; α-Terpineol, natural, ≥96%, FCC, FG; Geranyl formate; α-Pinene
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
-Cymene; (−)-Menthone; α-Terpineol, natural, ≥96%, FCC, FG; Terpinolene; β-Bourbonene; 1-Octen-3-ol; β-Caryophyllene; Linalool; α-Terpinene; (−)-Menthol
Cymene; 4,5,6,7-Tetrahydro-3,6-dimethylbenzofuran; Linalool; Menthol; Menthone; Menthyl acetate; Germacrene D; Bicyclogermacrene; Thymol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service