TZA000010
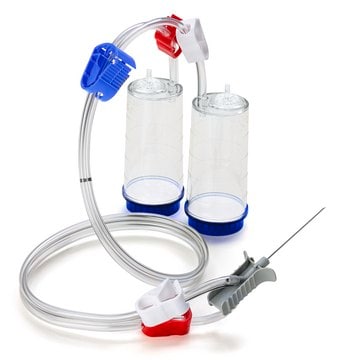
Steridilutor® NEO Device
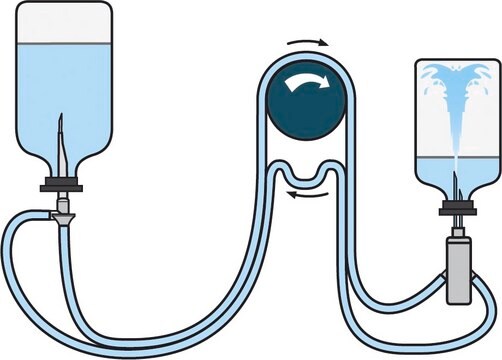
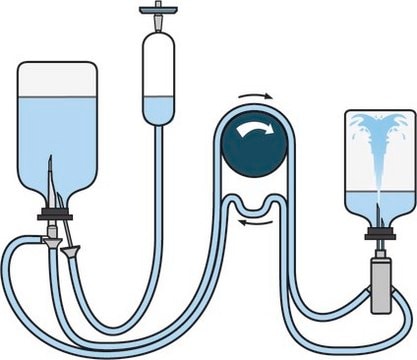
For liquid transfer from ampoules or vials to a diluent vial with septum pooling.
Synonym(s):
Steridilutor® NEO device for sterility testing
About This Item
Recommended Products
material
Nylon 66 adapter (for needle)
PVC tubing
stainless steel (for needle)
Quality Level
agency
EP (2.6.1)
JP (4.06)
USP 71
sterility
sterile; γ-irradiated
manufacturer/tradename
Steridilutor®
packaging
pkg of 10 blisters per box, Single packed
input
sample type pharmaceutical(s)
application(s)
pharmaceutical
sterility testing
compatibility
for use with Steritest® Symbio FLEX Pump Kit, 2 media (SYMBFLE01)
for use with Steritest® Symbio ISL Pump Kit, 2 media (SYMBISL01)
for use with Steritest® Symbio LFH Pump Kit (SYMBLFH01)
shipped in
ambient
General description
Application
Legal Information
Related product
Required but not provided
configured for
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
Regulatory compliant membrane filtration sterility testing devices to ensure the safety of your pharmaceutical products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service