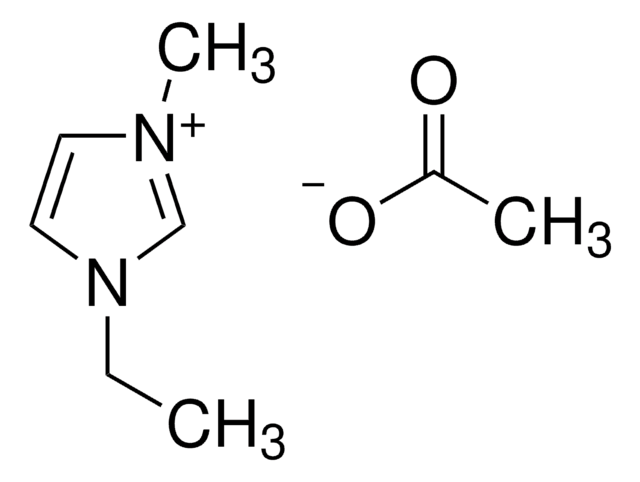
900787
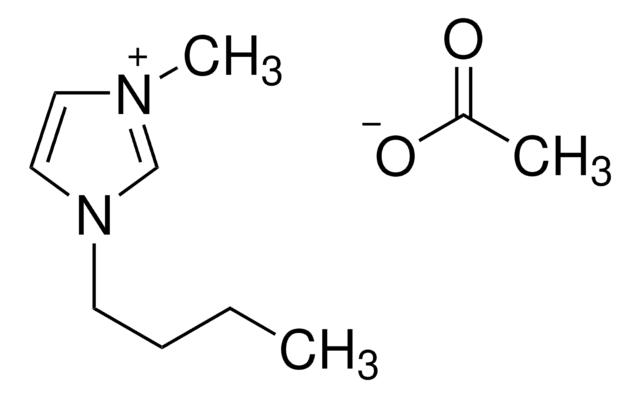
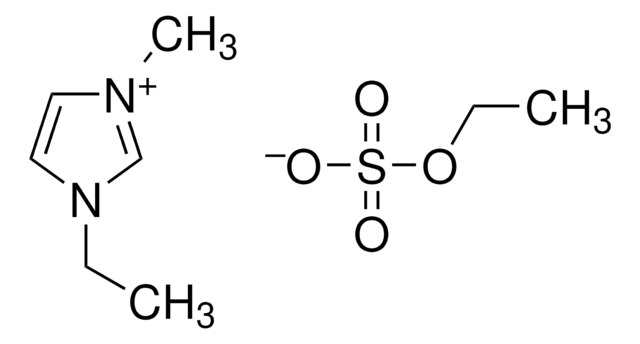
1-Ethyl-3-methylimidazolium acetate
≥98%
Synonym(s):
1-Methyl-3-ethylimidazolium acetate, 3-Ethyl-1-methylimidazolium acetate, EMIM Ac
About This Item
Recommended Products
Quality Level
assay
≥98%
form
liquid
greener alternative product characteristics
Catalysis
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤0.5% H2O
mp
>30 °C (product can occur as an undercooled melt)
density
1.101 g/cm3 at 20 °C
application(s)
battery manufacturing
greener alternative category
SMILES string
CC([O-])=O.CCn1cc[n+](C)c1
InChI
1S/C6H11N2.C2H4O2/c1-3-8-5-4-7(2)6-8;1-2(3)4/h4-6H,3H2,1-2H3;1H3,(H,3,4)/q+1;/p-1
InChI key
XIYUIMLQTKODPS-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
Related product
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2 - Skin Sens. 1B
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
327.2 °F - closed cup
flash_point_c
164 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Part III. The first readily biodegradable ionic liquids
Articles
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
Here, we present a short review of ionic liquid electrolytes used in state-of-the-art rechargeable batteries including high performance and low-cost aluminum batteries, non-flammable Li-based batteries, and high-cycling and stable dual-graphite batteries. We also outline the key issues explored so as to identify the future direction of IL development.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service