Kluczowe dokumenty
SML2416
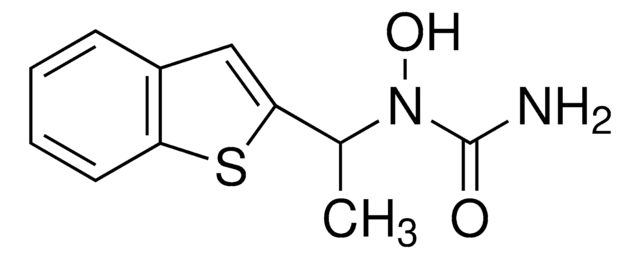
HET0016
≥95% (HPLC)
Synonim(y):
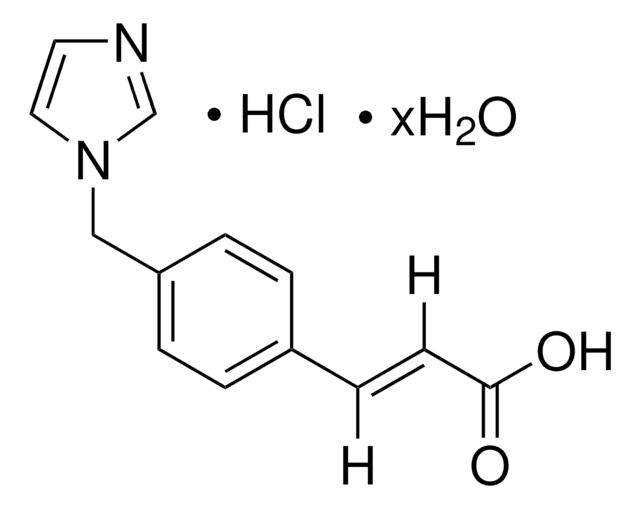
HET 0016, HET-0016, N′-(4-Butyl-2-methylphenyl)-N-hydroxymethanimidamide, N-(4-Butyl-2-methylphenyl)-N′-hydroxyformamidine, N-(4-Butyl-2-methylphenyl)-N′-hydroxymethanimidamide, N-Hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine
About This Item
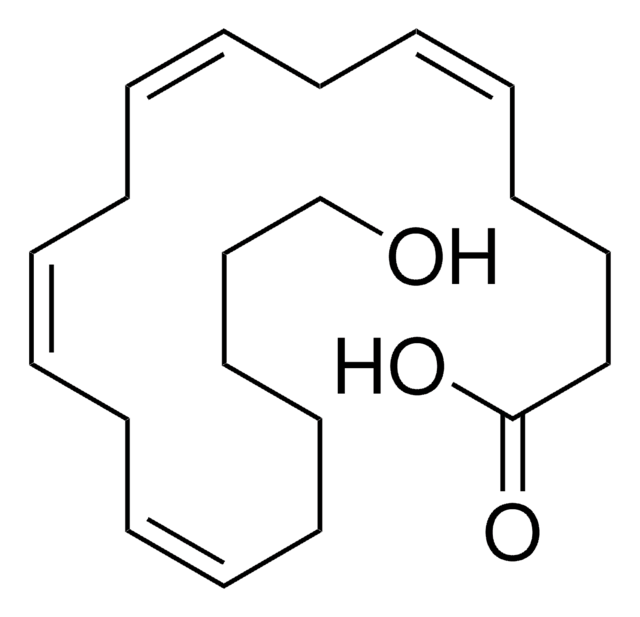
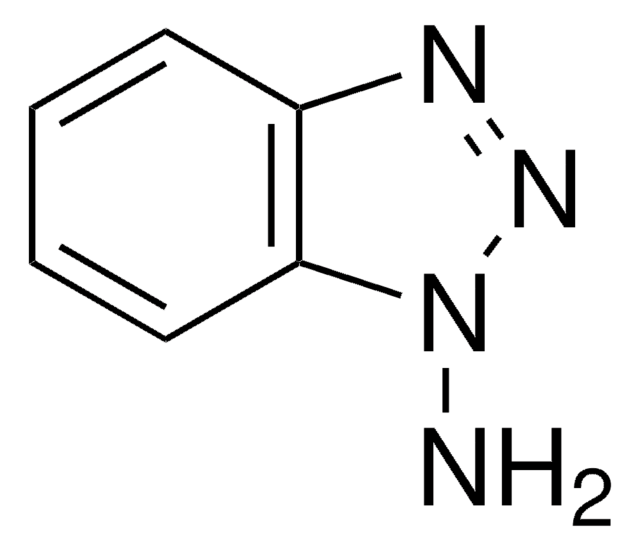
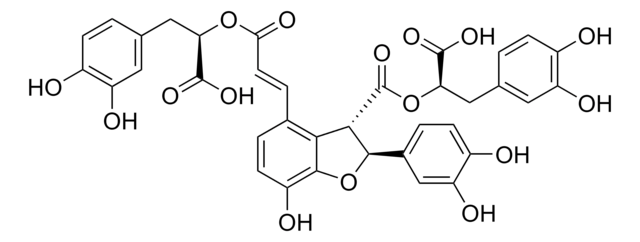
Polecane produkty
Próba
≥95% (HPLC)
Formularz
powder
warunki przechowywania
desiccated
kolor
white to beige
rozpuszczalność
DMSO: 2 mg/mL, clear
temp. przechowywania
−20°C
ciąg SMILES
CCCCC1=CC=C(C(C)=C1)/N=C/NO
InChI
1S/C12H18N2O/c1-3-4-5-11-6-7-12(10(2)8-11)13-9-14-15/h6-9,15H,3-5H2,1-2H3,(H,13,14)
Klucz InChI
LYNOGBKNFIHKLE-UHFFFAOYSA-N
Działania biochem./fizjol.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej