Wszystkie zdjęcia(3)
Kluczowe dokumenty
S4503
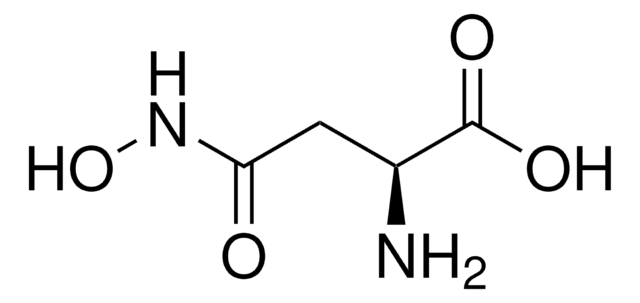
DL-Serine hydroxamate
≥97% (TLC), suitable for ligand binding assays
Synonim(y):
SHX
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C3H8N2O3
Numer CAS:
Masa cząsteczkowa:
120.11
Numer MDL:
Kod UNSPSC:
12352209
Identyfikator substancji w PubChem:
NACRES:
NA.26
Polecane produkty
Nazwa produktu
DL-Serine hydroxamate, seryl-tRNA synthetase inhibitor
Próba
≥97% (TLC)
Formularz
powder
metody
ligand binding assay: suitable
kolor
white to off-white
Zastosowanie
cell analysis
temp. przechowywania
−20°C
ciąg SMILES
NC(CO)C(=O)NO
InChI
1S/C3H8N2O3/c4-2(1-6)3(7)5-8/h2,6,8H,1,4H2,(H,5,7)
Klucz InChI
LELJBJGDDGUFRP-UHFFFAOYSA-N
Zastosowanie
Serine has been used as an inhibitor of seryl-tRNA synthetase. DL-Serine hydroxamate is used to induce metabolic synthesis of guanosine 3′-diphosphate 5′-diphosphate (ppGpp) in E. coli by amino acid starvation. It is also used to synchronize cell cycle in E. coli cultures by inhibition of tRNA charging.
Działania biochem./fizjol.
Serine is involved in the one-carbon unit metabolism. It is associated with the biosynthesis of cysteine, ceramide, phosphatidylserine, purine and pyrimidine. In bacteria, it participates in tryptophan synthesis. Gluconeogenesis, one of the important biochemical processes, involves serine, particularly in ruminants. Protein phosphorylation is one such event that utilizes serine. Glycine, a metabolic product of serine, serves as an antioxidant and a neurotransmitter. D-serine is known to activate the N-methyl-D-aspartate (NMDA) receptors of the brain. Serine hydroxamate, a structural analogue of serine prevents seryl-tRNA (transfer ribonucleic acid) charging and thereby decreases phospholipid and nucleic acid synthesis in Escherichia coli.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Olaf Brockmann-Gretza et al.
BMC genomics, 7, 230-230 (2006-09-12)
The stringent response is the initial reaction of microorganisms to nutritional stress. During stringent response the small nucleotides (p)ppGpp act as global regulators and reprogram bacterial transcription. In this work, the genetic network controlled by the stringent response was characterized
Dao Nguyen et al.
Science (New York, N.Y.), 334(6058), 982-986 (2011-11-19)
Bacteria become highly tolerant to antibiotics when nutrients are limited. The inactivity of antibiotic targets caused by starvation-induced growth arrest is thought to be a key mechanism producing tolerance. Here we show that the antibiotic tolerance of nutrient-limited and biofilm
H J Cha et al.
Applied and environmental microbiology, 65(2), 409-414 (1999-01-30)
We constructed and characterized three stress probe plasmids which utilize a green fluorescent protein as a noninvasive reporter in order to elucidate Escherichia coli cellular stress responses in quiescent or resting cells. Cellular stress levels were easily detected by fusing
Yuki Matsumoto et al.
BMC genomics, 14, 808-808 (2013-11-21)
Cell growth rate reflects an organism's physiological state and largely relies on the ability of gene expression to respond to the environment. The relationship between cellular growth rate and gene expression remains unknown. Growth rate-coordinated changes in gene expression were
B Belitsky et al.
The Journal of biological chemistry, 257(9), 4677-4679 (1982-05-10)
Lack of three different amino acids or treatment with the analogue DL-serine hydroxamate does not induce the accumulation of ppGpp and pppGpp, the 3'-pyrophosphates of GDP and GTP, respectively, in Rhizobium meliloti strain 41. Surprisingly, RNA accumulation is controlled under
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej