Kluczowe dokumenty
MTOX1020
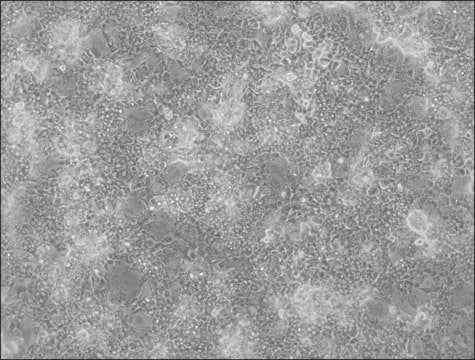
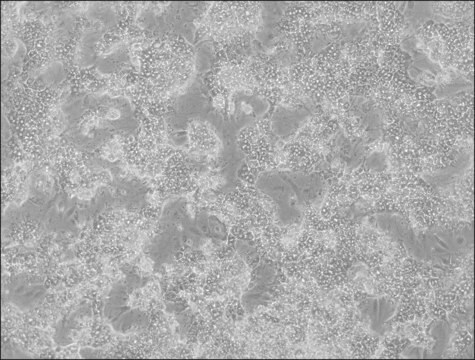
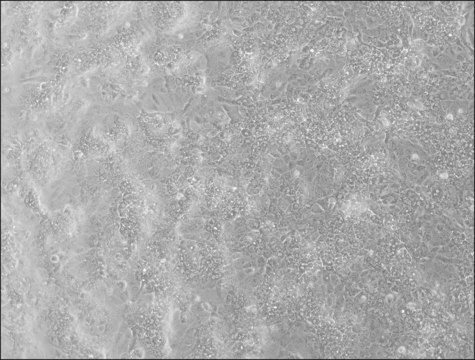
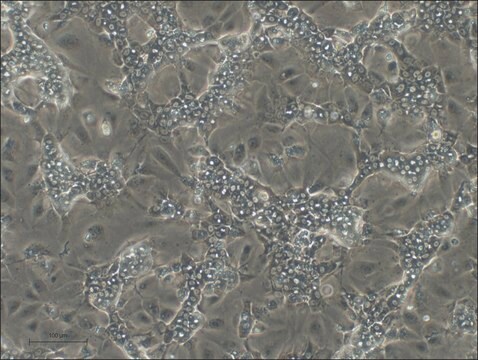
HepaRG™ Cells MRP2 (-/-)
human female liver (hepatocarcinoma and hepatitis C tumor)
About This Item
Polecane produkty
Nazwa produktu
HepaRG™ Cells MRP2 (-/-), one vial
pochodzenie biologiczne
human female liver (hepatocarcinoma and hepatitis C tumor)
Poziom jakości
Formularz
liquid
numer dostępu OMIM
temp. przechowywania
−196°C
informacje o genach
human ... ABCC2(1244)
Opis ogólny
Zastosowanie
Cechy i korzyści
- The frame-shift mutation of ABCC2 gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of transport of selective substrates in sandwich culture assay.
Jakość
Informacje prawne
Exhibit 2: HepaRG limited use license
Oświadczenie o zrzeczeniu się odpowiedzialności
Kod klasy składowania
12 - Non Combustible Liquids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Produkty
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej