MSQC3
SILu™MAB Stable-Isotope Labeled Universal Monoclonal Antibody Standard human
recombinant, expressed in CHO cells
Synonim(y):
IgG znakowane stabilnym izotopem, Standard spektrometrii masowej, IgG, Wzorzec spektrometrii mas, immunoglobulina-G
About This Item
Polecane produkty
rekombinowane
expressed in CHO cells
Poziom jakości
Próba
≥90% (SDS-PAGE)
opakowanie
vial of 100 μg (± 10% Lot-specific vial content given on certificate of analysis)
Warunki transportu
wet ice
temp. przechowywania
−20°C
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Zastosowanie
Cechy i korzyści
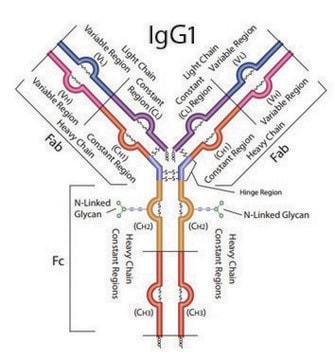
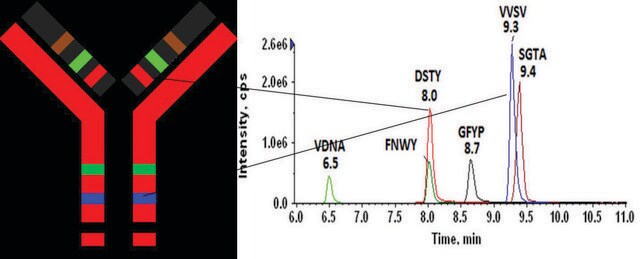
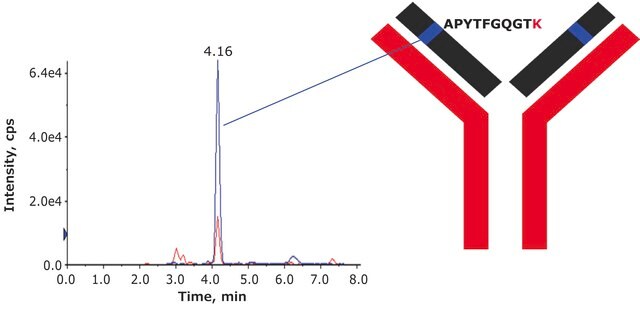
DTLMISRHeavy Chain (IgG1, IgG2, IgG3, IgG4)
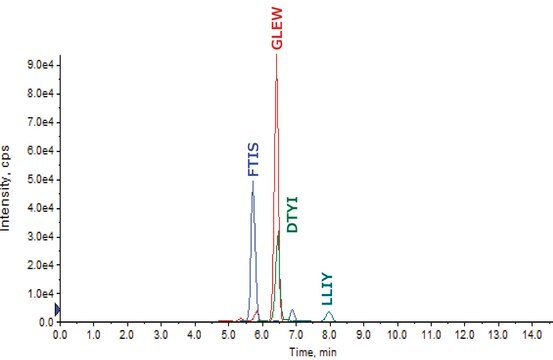
FNWYVDGVEVHNAKHeavy Chain (IgG1)
VVSVLTVLHQDWLNGKHeavy Chain (IgG1, IgG3, IgG4)
NQVSLTCLVKHeavy Chain (IgG1, IgG2, IgG3, IgG4)
GFYPSDIAVEWESNGQPENNYKHeavy Chain (IgG1, IgG4)
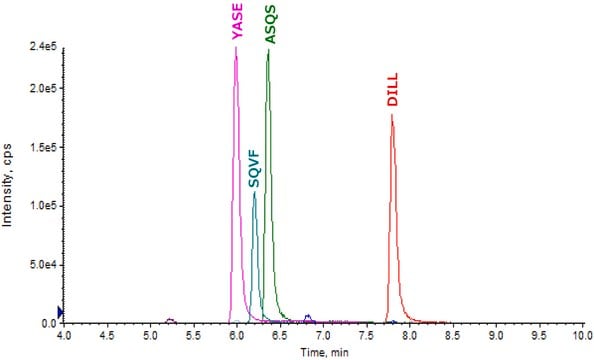
AGVETTTPSKLight Chain (lambda)
YAASSYLSLTPEQWKLight Chain (lambda)
- SILuMab yielded reproducible, linear curves from 0.1 μg/mL to 1000 μg/mL without enrichment or depletion.
- Good agreement was observed between multiple peptides derived from the same target.
- Label incorporation was determined to be >98% by mass spectrometry.
- Sequence coverage was confirmed by peptide mapping.
Postać fizyczna
Uwaga dotycząca przygotowania
Rekonstytucja
Procedure
- Briefly centrifuge the vial at ~10,000 x g to collect the product at the bottom of the vial.
- Add 500 μL of purified water containing 0.1% formic acid to the vial.
- Mix the contents by gently inverting the vial a minimum of 5 times.
- Allow the vial to stand at room temperature for a minimum of 15 minutes and repeat mixing by inversion.
Komentarz do analizy
EVQLVESGGGLVQPGGSLRLSCVASGFTLNNYDMHWVRQGIGKGLEWVSKI
GTAGDRYYAGSVKGRFTISRENAKDSLYLQMNSLRVGDAAVYYCARGAGRW
APLGAFDIWGQGTMVTVSS|ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
RDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
SILuMab Light Chain
QSALTQPRSVSGSPGQSVTISCTGTSSDIGGYNFVSWYQQHPGKAPKLMIY
DATKRPSGVPDRFSGSKSGNTASLTISGLQAEDEADYYCCSYAGDYTPGV
VFGGGTKLTVL|GQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTV
AWKADSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQ
VTHEGSTVEKTVAPTECS
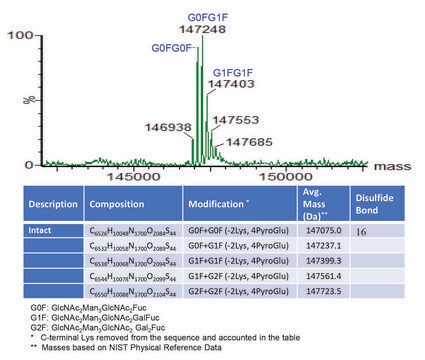
Target overlap areas are underlined
Intact heavy and light chains (FASTA file)
Quantitative
MRM settings provided (Skyline, xls)
Informacje prawne
produkt powiązany
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Powiązane treści
Odkryj naszą szeroką gamę produktów do analizy masy przeciwciał monoklonalnych w stanie nienaruszonym, w tym kolumny wykluczające (SEC), kolumny jonowymienne, kolumny fazy odwróconej, bufory HPLC, matryce i wzorce MALDI, rozpuszczalniki o wysokiej czystości, odczynniki, narzędzia do przygotowywania próbek białek i certyfikowane materiały referencyjne.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej