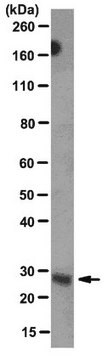
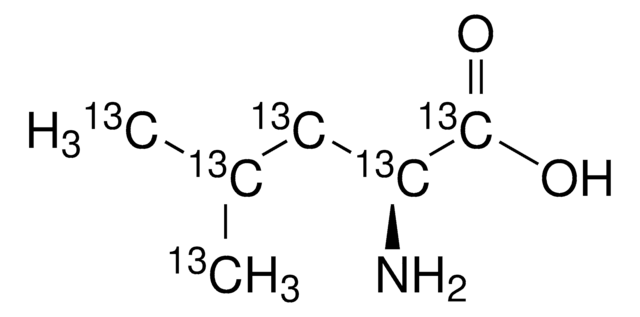
Kluczowe dokumenty
MAK054
α-Ketoglutarate Assay Kit
sufficient for 100 colorimetric or fluorometric tests
About This Item
Polecane produkty
zastosowanie
sufficient for 100 colorimetric or fluorometric tests
metoda wykrywania
colorimetric
fluorometric
powiązane choroby
cancer
temp. przechowywania
−20°C
Opis ogólny
Zastosowanie
Przydatność
Zasada
produkt powiązany
zastąpiony przez
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Resp. Sens. 1 - Skin Sens. 1
Kod klasy składowania
10 - Combustible liquids
Temperatura zapłonu (°F)
188.6 °F - closed cup
Temperatura zapłonu (°C)
87 °C - closed cup
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Produkty
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Protokoły
We describe here a rapid and sensitive method to separate and measure D-2-OHG and L-2-OHG enantiomers using high-resolution mass spectrometry (HRMS) detection.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej