Kluczowe dokumenty
K1876
Kanamycin disulfate salt from Streptomyces kanamyceticus
aminoglycoside antibiotic
Synonim(y):
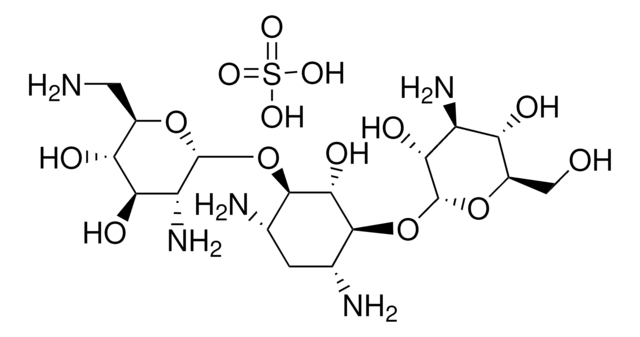
Kanamycin Disulfate Salt, Kanamycin disulfate, O-3-amino-3-deoxy-alpha-D-glucopyranosyl-(1->6)-O-(6-amino-6-deoxy-alpha-D-glucopyranosyl-(1->4))-2-deoxy-D-Streptamine sulfate (1:2) (salt), Kanamycin A, Kanamycin acid sulfate
About This Item
Polecane produkty
pochodzenie biologiczne
Streptomyces kanamyceticus
Poziom jakości
Postać
powder
siła działania
~650 μg per mg
zanieczyszczenia
≤4% Kanamycin B
kolor
white to off-white
rozpuszczalność
H2O: 10 mg/mL
spektrum działania antybiotyku
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
mycoplasma
Tryb działania
protein synthesis | interferes
ciąg SMILES
OS(O)(=O)=O.OS(O)(=O)=O.NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C18H36N4O11.2H2O4S/c19-2-6-10(25)12(27)13(28)18(30-6)33-16-5(21)1-4(20)15(14(16)29)32-17-11(26)8(22)9(24)7(3-23)31-17;2*1-5(2,3)4/h4-18,23-29H,1-3,19-22H2;2*(H2,1,2,3,4)/t4-,5+,6-,7-,8+,9-,10-,11-,12+,13-,14-,15+,16-,17-,18-;;/m1../s1
Klucz InChI
OGTKIXVMLDAMNU-KNQICTBBSA-N
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Mode of Resistance: Aminoglycoside-modifying enzymes (including acetyltransferase, phosphotransferase, nucleotidyltransferase) can alter this antibiotic, preventing its interaction with ribosomes.
Antimicrobial Spectrum: Kanamycin sulfate is effective against gram-negative and gram-postiive bacteria, and mycoplasma.
Przestroga
Uwaga dotycząca przygotowania
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Repr. 1B
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej