D5314
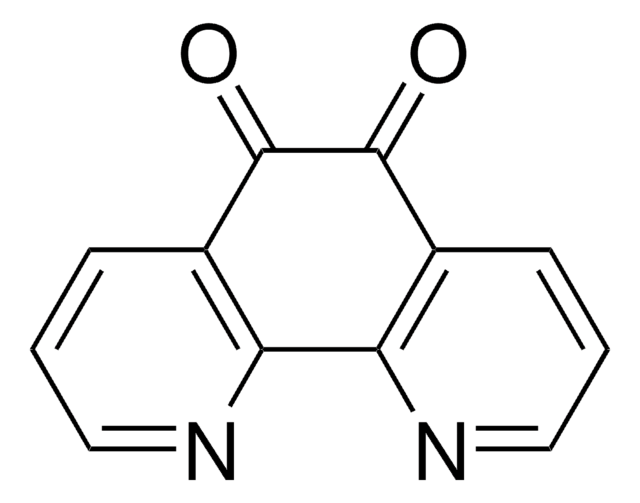
DPQ
≥98% (HPLC), solid
Synonim(y):
3,4-Dihydro-5-[4-(1-piperidinyl)butoxyl]-1(2H)-isoquinolinone
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C18H26N2O2
Numer CAS:
Masa cząsteczkowa:
302.41
Numer MDL:
Kod UNSPSC:
12352200
NACRES:
NA.77
Polecane produkty
pochodzenie biologiczne
synthetic (organic)
Poziom jakości
Próba
≥98% (HPLC)
Formularz
solid
mp
107-109 °C
rozpuszczalność
DMSO: 1 mg/mL, clear, colorless to faintly yellow
temp. przechowywania
2-8°C
InChI
1S/C18H26N2O2/c21-18-16-7-6-8-17(15(16)9-10-19-18)22-14-5-4-13-20-11-2-1-3-12-20/h6-8H,1-5,9-14H2,(H,19,21)
Klucz InChI
RVOUDNBEIXGHJY-UHFFFAOYSA-N
Zastosowanie
DPQ has been used as a PARP1 (poly(ADP-ribose) polymerase 1) inhibitor in in vivo studies to determine the loss of γ-H2AX (H2A histone family member X) upon irradiation.
Działania biochem./fizjol.
3,4-Dihydro-5-[4-(1-piperidinyl)butoxyl]-1(2H)-isoquinolinone (DPQ) is known to decrease the PARP 1 (poly(ADP-ribose) polymerase 1) mediated apoptosis under the influence of ischemia. It is considered as more effective inhibitor than the traditionally used PARP1 inhibitor 3-aminobenzamide.
DPQ is a very potent poly(ADP-ribose) polymerase (PARP) inhibitor.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Parp1-XRCC1 and the repair of DNA double strand breaks in mouse round spermatids
Ahmed EA, et al.
Mutation Research, 683(1), 84-90 (2010)
Ujval Anilkumar et al.
PloS one, 12(11), e0188343-e0188343 (2017-11-18)
Cell death induced by excessive glutamate receptor overactivation, excitotoxicity, has been implicated in several acute and chronic neurological disorders. While numerous studies have demonstrated the contribution of biochemically and genetically activated cell death pathways in excitotoxic injury, the factors mediating
M J Suto et al.
Anti-cancer drug design, 6(2), 107-117 (1991-05-01)
A series of dihydroisoquinolinones, formally rigid analogs of 3-substituted benzamides, and a series of 2,3-disubstituted benzamides were synthesized and evaluated as inhibitors of poly(ADP-ribose) polymerase. The results indicated that the orientation of the amide with respect to the substituent on
Advances in Neonatal Care : Official Journal of the National Association of Neonatal Nurses, 29-29 (2012)
M J Eliasson et al.
Nature medicine, 3(10), 1089-1095 (1997-10-23)
Nitric oxide (NO) and peroxynitrite, formed from NO and superoxide anion, have been implicated as mediators of neuronal damage following focal ischemia, but their molecular targets have not been defined. One candidate pathway is DNA damage leading to activation of
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej