Kluczowe dokumenty
A8001
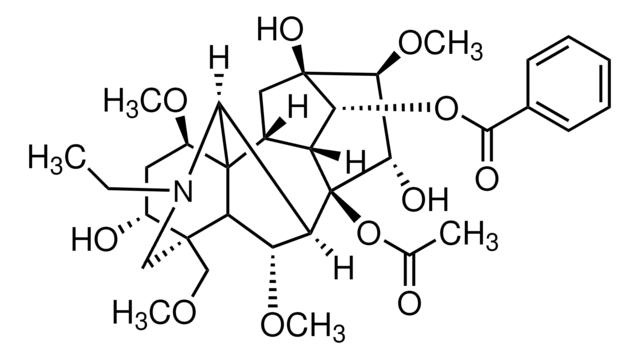
Aconitine
≥95% (HPLC), crystalline
Synonim(y):
Acetylbenzoylaconine
About This Item
Polecane produkty
Poziom jakości
Próba
≥95% (HPLC)
Formularz
crystalline
kolor
white
rozpuszczalność
H2O: 0.3 mg/mL
ethanol: 35 mg/mL
ciąg SMILES
CCN1C[C@]2(COC)[C@H](O)C[C@@H](OC)C34C5C[C@]6(O)[C@@H](OC)[C@H](O)[C@@](OC(C)=O)(C5[C@H]6OC(=O)c7ccccc7)C([C@H](OC)C23)C14
InChI
1S/C34H47NO11/c1-7-35-15-31(16-41-3)20(37)13-21(42-4)33-19-14-32(40)28(45-30(39)18-11-9-8-10-12-18)22(19)34(46-17(2)36,27(38)29(32)44-6)23(26(33)35)24(43-5)25(31)33/h8-12,19-29,37-38,40H,7,13-16H2,1-6H3/t19-,20-,21-,22-,23+,24+,25?,26?,27+,28-,29+,31+,32-,33?,34-/m1/s1
Klucz InChI
XFSBVAOIAHNAPC-VBUFWTEXSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
- to study its cardiotoxic effects along the pericardium meridian (PM) on cardiac rhythm in rabbits
- as a standard in high-performance thin layer chromatography (HPTLC) fingerprinting method
- in the aconitine-based lipo-alkaloids semi-synthesis
Działania biochem./fizjol.
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 1 Oral - Acute Tox. 2 Inhalation
Kod klasy składowania
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej