Wszystkie zdjęcia(1)
Kluczowe dokumenty
A6362
Alpha-lytic protease
Synonim(y):
Alpha-lytic endopeptidase, alphaLP
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Polecane produkty
pochodzenie biologiczne
bacterial
Formularz
liquid
aktywność właściwa
≥0.0005 U/mg
masa cząsteczkowa
19,860 Da
optymalne pH
5.0(storage)
7.5(activity)
pI
9.69
Warunki transportu
dry ice
temp. przechowywania
−70°C
Opis ogólny
Alpha-lytic protease (aLP) is an alternative specificity protease for proteomics applications. This protease cleaves after T, A, S, and V residues. It generates peptides of similar average length as trypsin.
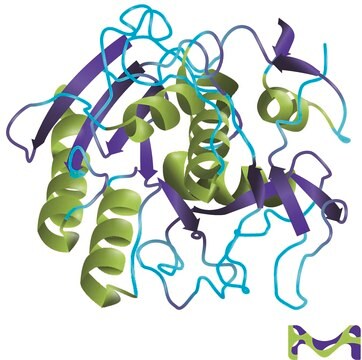
aLP was first isolated from the myxobacterium Lysobacter enzymogenes. The pro-form of aLP is 397 amino acids long. In its mature form, aLP is 198 amino acids long. Its tertiary structural core resembles those of pancreatic serine proteases.
Crystal structure studies of aLP have been reported. Several studies are available on the active site and catalytic mechanism of aLP. The role of the pro-region in the activation, secretion and folding of aLP has been studied.
The activity of aLP in the presence of various solution components is as follows:
aLP was first isolated from the myxobacterium Lysobacter enzymogenes. The pro-form of aLP is 397 amino acids long. In its mature form, aLP is 198 amino acids long. Its tertiary structural core resembles those of pancreatic serine proteases.
Crystal structure studies of aLP have been reported. Several studies are available on the active site and catalytic mechanism of aLP. The role of the pro-region in the activation, secretion and folding of aLP has been studied.
The activity of aLP in the presence of various solution components is as follows:
- 0.1% sodium deoxycholate: ~1.75-fold enhanced activity
- 1.0% sodium deoxycholate: ~60% activity
- 0.1% SDS: ~50% activity
- 1.0% SDS: ~40% activity
- 1 M guanidine HCl: ~20% activity
- 4 M guanidine HCl: ~1% activity (essentially inactivated)
Definicja jednostki
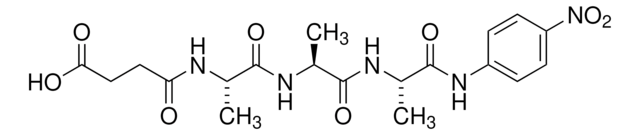
One unit will produce one mmole of p-nitroaniline per minute from N-succinyl-Ala-Ala-Ala-PNA at 25 °C at pH 7.5
Postać fizyczna
Supplied as a solution in 10 mM sodium acetate buffer, pH 5.0.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
12 - Non Combustible Liquids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Lot/Batch Number
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
C A Bauer et al.
European journal of biochemistry, 120(2), 289-294 (1981-11-01)
The active site of alpha-lytic protease has been studied with a number of synthetic peptides and compared with similar data published for elastase. The kinetic data indicate that the active site of alpha-lytic protease extends over at least six subsites
M Fujinaga et al.
Journal of molecular biology, 184(3), 479-502 (1985-08-05)
The structure of alpha-lytic protease, a serine protease produced by the bacterium Lysobacter enzymogenes, has been refined at 1.7 A resolution. The conventional R-factor is 0.131 for the 14,996 reflections between 8 and 1.7 A resolution with I greater than
Molecular structure of the alpha-lytic protease from Myxobacter 495 at 2.8 Angstroms resolution.
G D Brayer et al.
Journal of molecular biology, 131(4), 743-775 (1979-07-15)
Jesse G Meyer et al.
Molecular & cellular proteomics : MCP, 13(3), 823-835 (2014-01-16)
Bottom-up proteomics studies traditionally involve proteome digestion with a single protease, trypsin. However, trypsin alone does not generate peptides that encompass the entire proteome. Alternative proteases have been explored, but most have specificity for charged amino acid side chains. Therefore
The α-Lytic Protease.
Whitaker, D.R.
Methods in Enzymology, 19, 599-613 (1970)
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej