Key Documents
A0783
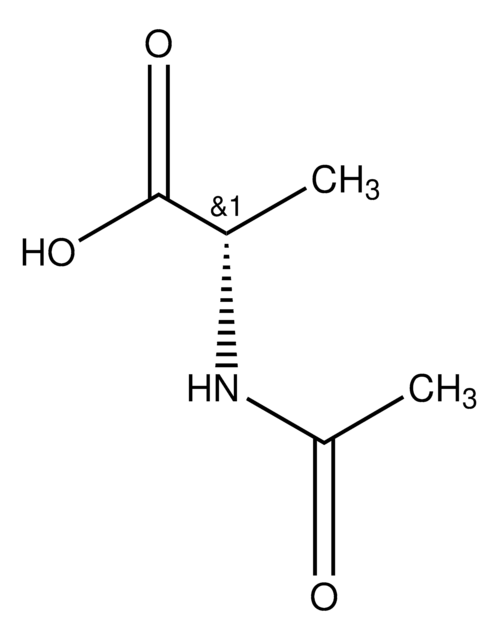
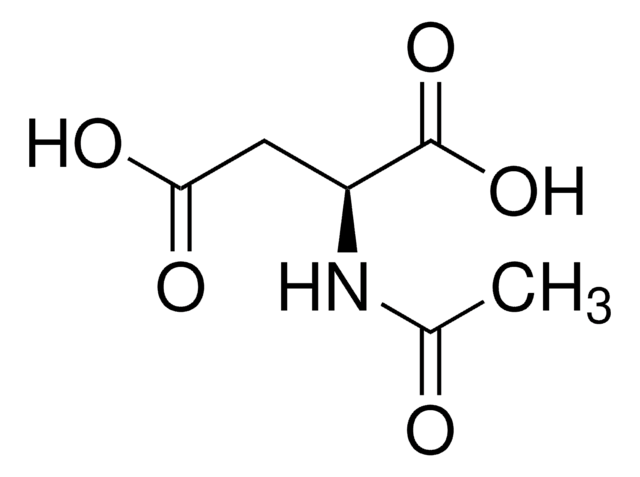
N-Acetyl-L-proline
≥98% (TLC), suitable for ligand binding assays
About This Item
Polecane produkty
product name
N-Acetyl-L-proline,
Próba
≥98% (TLC)
Poziom jakości
Postać
powder
metody
ligand binding assay: suitable
kolor
white
temp. przechowywania
2-8°C
ciąg SMILES
CC(=O)N1CCC[C@H]1C(O)=O
InChI
1S/C7H11NO3/c1-5(9)8-4-2-3-6(8)7(10)11/h6H,2-4H2,1H3,(H,10,11)/t6-/m0/s1
Klucz InChI
GNMSLDIYJOSUSW-LURJTMIESA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Działania biochem./fizjol.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej