Kluczowe dokumenty
94335
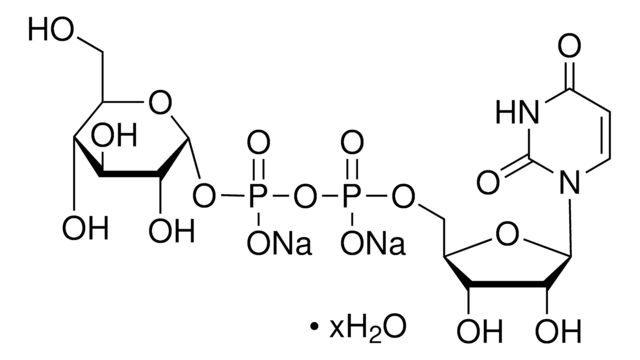
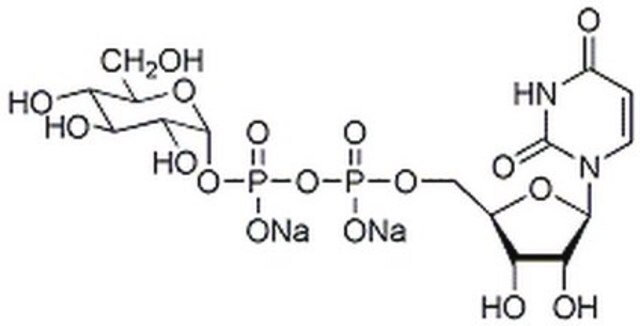
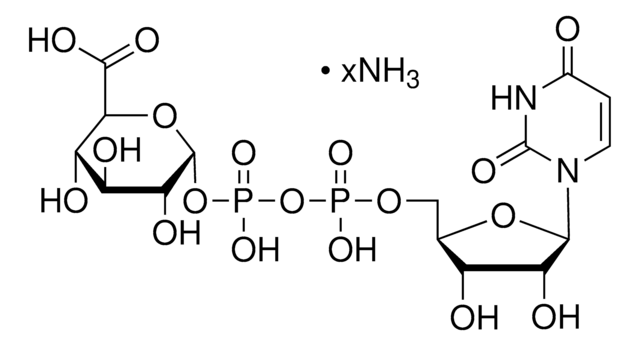
Uridine 5′-diphosphoglucose disodium salt
≥98.0% (HPLC)
Synonim(y):
UDP-D-Glucose disodium salt, UDPG, UDP-glc, Uridine-diphosphate-glucose disodium salt, Uridine[5’]diphospho[1]-α-D-glucopyranose disodium salt
Wybierz wielkość
Wybierz wielkość
About This Item
Polecane produkty
pochodzenie biologiczne
Saccharomyces cerevisiae
Próba
≥98.0% (HPLC)
Formularz
powder
zanieczyszczenia
≤5% solvent
≤8.5% water
rozpuszczalność
H2O: 50 mg/mL, clear
temp. przechowywania
−20°C
ciąg SMILES
[Na+].[Na+].OC[C@H]1O[C@H](OP([O-])(=O)OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)N3C=CC(=O)NC3=O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C15H24N2O17P2.2Na/c18-3-5-8(20)10(22)12(24)14(32-5)33-36(28,29)34-35(26,27)30-4-6-9(21)11(23)13(31-6)17-2-1-7(19)16-15(17)25;;/h1-2,5-6,8-14,18,20-24H,3-4H2,(H,26,27)(H,28,29)(H,16,19,25);;/q;2*+1/p-2/t5-,6-,8-,9-,10+,11-,12-,13-,14-;;/m1../s1
Klucz InChI
PKJQEQVCYGYYMM-QBNUFUENSA-L
Opis ogólny
Zastosowanie
- as a substrate in the enzymatic production of glycosides and their detection by liquid chromatography-mass spectrometry.
- as a substrate in the substrate screening and binding affinity measurements of the human CMP-Sia transporter.
Działania biochem./fizjol.
Inne uwagi
zastąpiony przez
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wykazy regulacyjne
Wykazy regulacyjne dotyczą głównie produktów chemicznych. Można w nich podawać ograniczoną liczbę informacji na temat produktów niechemicznych. Brak wpisu oznacza, że żaden ze składników nie znajduje się w wykazie. Użytkownik odpowiada za zagwarantowanie bezpiecznego i zgodnego z prawem stosowania produktu.
EU REACH Annex XVII (Restriction List)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej