Kluczowe dokumenty
72987
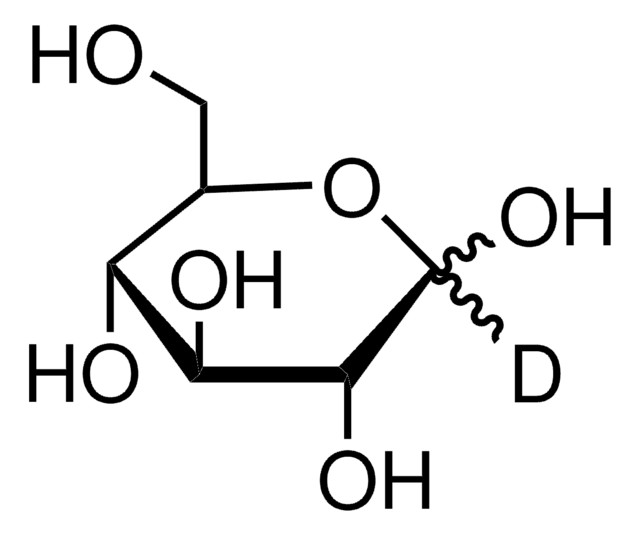
2-Deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-glucose
≥97% (HPLC)
Synonim(y):
2-NBDG, 2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose
About This Item
Polecane produkty
pochodzenie biologiczne
synthetic
Poziom jakości
Próba
≥97% (HPLC)
Formularz
powder
fluorescencja
λex 494 nm; λem 551 nm in DMSO
Zastosowanie
cell analysis
temp. przechowywania
−20°C
ciąg SMILES
OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](Nc1ccc([N+]([O-])=O)c2nonc12)C=O
InChI
1S/C12H14N4O8/c17-3-6(11(20)12(21)8(19)4-18)13-5-1-2-7(16(22)23)10-9(5)14-24-15-10/h1-3,6,8,11-13,18-21H,4H2/t6-,8+,11+,12+/m0/s1
Klucz InChI
QUTFFEUUGHUPQC-ILWYWAAHSA-N
Opis ogólny
Zastosowanie
Opakowanie
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej