Y0000319
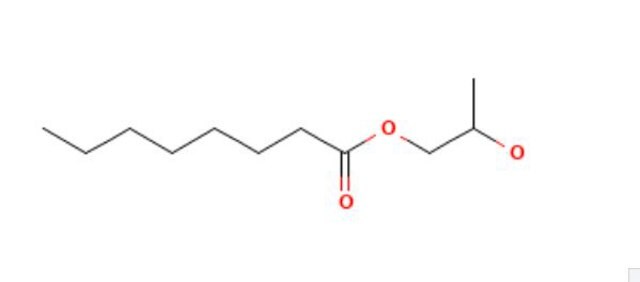
Propylene glycol monolaurate
European Pharmacopoeia (EP) Reference Standard
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C15H30O3
Numer CAS:
Masa cząsteczkowa:
258.40
Numer WE:
Kod UNSPSC:
41116107
NACRES:
NA.24
Polecane produkty
klasa czystości
pharmaceutical primary standard
rodzina API
propylene glycol
producent / nazwa handlowa
EDQM
Zastosowanie
pharmaceutical (small molecule)
format
neat
temp. przechowywania
2-8°C
InChI
1S/C15H30O3/c1-3-4-5-6-7-8-9-10-11-12-15(17)18-13-14(2)16/h14,16H,3-13H2,1-2H3
Klucz InChI
BHIZVZJETFVJMJ-UHFFFAOYSA-N
Opis ogólny
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Zastosowanie
Propylene glycol monolaurate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Opakowanie
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Inne uwagi
Sales restrictions may apply.
This page may contain text that has been machine translated.
produkt powiązany
Numer produktu
Opis
Cennik
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Lot/Batch Number
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Rana Abu-Huwaij et al.
Drug development and industrial pharmacy, 33(4), 437-448 (2007-05-25)
The aim of this study was to develop a controlled release buccal mucoadhesive delivery system for systemic delivery of lidocaine hydrochloride as a model drug. In vitro release and buccal permeation as well as in vivo permeation of LDHCL patches
Sang-Chul Shin et al.
Archives of pharmacal research, 29(10), 928-933 (2006-11-24)
Percutaneous delivery of NSAIDs has advantages of avoiding hepatic first pass effect and delivering the drug for extended period of time at a sustained, concentrated level at the inflammation site that mainly acts at the joint and the related regions.
B R Jasti et al.
The journal of investigative dermatology. Symposium proceedings, 3(2), 128-130 (1998-09-12)
Excipients are often used in transdermal formulations to overcome the formidable barrier offered by the epidermis in order to achieve the target flux. In this study we describe the use of frequency-domain fluorescence spectroscopy to characterize the effect of two
Jian Meng et al.
Drug development and industrial pharmacy, 33(9), 927-931 (2007-09-25)
Self-microemulsifying drug delivery systems (SMEDDS) are useful to improve the bioavailability of poorly water-soluble drugs by increasing their apparent solubility through solubilization. However, very few studies, to date, have systematically examined the level of drug apparent solubility in o/w microemulsion
Archita Patel et al.
Current drug delivery, 12(6), 745-760 (2015-03-04)
The solid-self nanoemulsifying drug delivery system (S-SNEDDS) of Amiodarone hydrochloride (AH) was prepared and evaluated. AH exhibits poor aqueous solubility (0.3-0.5 mg/ml) and therefore variable oral bioavailability. Capmul MCM, Cremophor RH-40 and Propylene glycol were identified as oil, surfactant and
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej