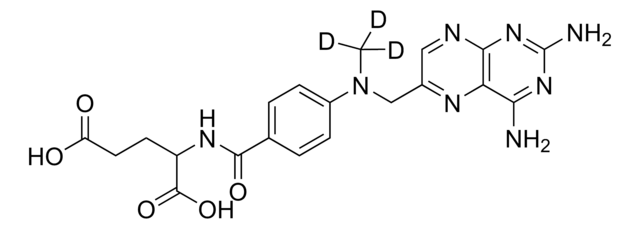
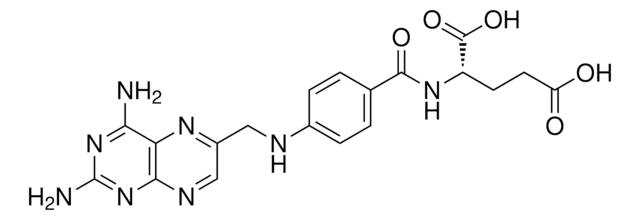
Kluczowe dokumenty
M9929
Methotrexate
meets USP testing specifications
Synonim(y):
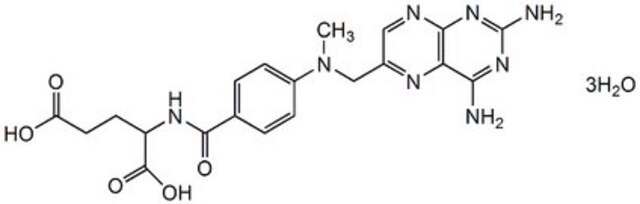
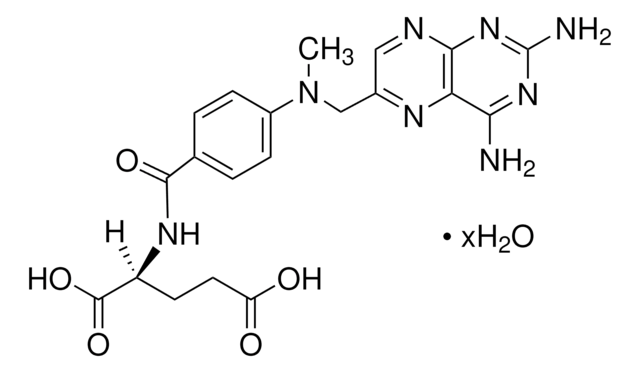
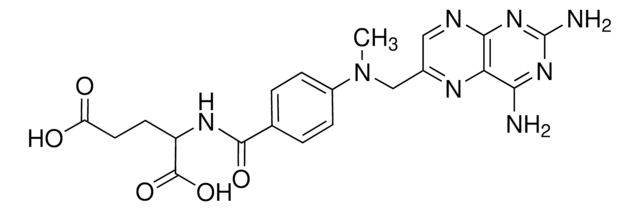
Methotrexate hydrate, 4-Amino-10-methylfolic acid hydrate, L-4-Amino-N10-methylpteroylglutamic acid hydrate, L-Amethopterin hydrate, Antifolan hydrate, MTX hydrate, Methylaminopterin hydrate
About This Item
Polecane produkty
pochodzenie biologiczne
synthetic
Poziom jakości
agency
USP/NF
meets USP testing specifications
Próba
98.0-102.0%
Formularz
powder
warunki przechowywania
(Tightly closed. Dry. Keep in a well-ventilated place. )
metody
activity assay: suitable
zanieczyszczenia
≤0.1% Residue on ignition (Ash)
≤12.0% water (Karl Fischer)
kolor
yellow to orange-yellow
mp
185-204 °C (365 - 399 °F)
rozpuszczalność
water: insoluble
spektrum działania antybiotyku
parasites
Zastosowanie
metabolomics
microbiology
Tryb działania
DNA synthesis
temp. przechowywania
−20°C
ciąg SMILES
[H]O[H].CN(Cc1cnc2nc(N)nc(N)c2n1)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O
InChI
1S/C20H22N8O5.H2O/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30;/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27);1H2/t13-;/m0./s1
Klucz InChI
FPJYMUQSRFJSEW-ZOWNYOTGSA-N
informacje o genach
human ... DHFR(1719) , DHFRP1(573971) , FPGS(2356) , SLC19A1(6573) , TYMS(7298)
mouse ... Dhfr(13361)
rat ... Dhfr(24312)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
Działania biochem./fizjol.
Inne uwagi
produkt powiązany
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Oral - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Separation of Methotrexate (MTX)-PG; Methotrexate (MTX)-PG2; Methotrexate (MTX)-PG3 ; Methotrexate (MTX)-PG4; Methotrexate (MTX)-PG5; Methotrexate (MTX)-PG6; Methotrexate (MTX)-PG7
This article reviews some of our newest and most innovative technologies and their specific applications toward cancer research. It describes how complex the disease of cancer is, and how difficult it is to identify one topic that is completely unrelated to any other.
This issue of Biofiles reviews some of our newest and most innovative technologies and their specific applications toward cancer research. In preparing this issue of Biofiles, one is reminded how complex the disease of cancer is, and how difficult it is to identify one topic that is completely unrelated to any other.
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej