Kluczowe dokumenty
61188
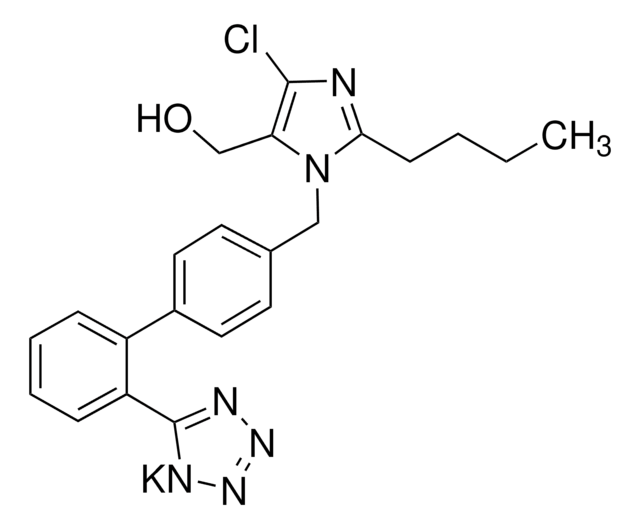
Losartan potassium
Synonim(y):
2-Butyl-4-chloro-1-{[2′-(1H-tetrazol-5-yl)(1,1′-biphenyl)-4-yl]methyl}-1H-imidazole-5-methanol monopotassium salt, 2-butyl-4-chloro-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]- 1H-Imidazole-5-methanol potassium salt, DuP 753, MK 954, Potassium 5-(4′-((2-butyl-4-chloro-5-(hydroxymethyl)-1H-imidazol-1-yl)methyl)-[1,1′-biphenyl]-2-yl)tetrazol-1-ide
About This Item
Polecane produkty
Formularz
powder or crystals
Poziom jakości
okres trwałości
limited shelf life, expiry date on the label
metody
HPLC: suitable
gas chromatography (GC): suitable
zanieczyszczenia
≤0.5% water
Zastosowanie
forensics and toxicology
pharmaceutical (small molecule)
veterinary
ciąg SMILES
CCCCc1nc(Cl)c(CO)n1Cc2ccc(cc2)-c3ccccc3-c4nnnn4[K]
InChI
1S/C22H22ClN6O.K/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22;/h4-7,9-12,30H,2-3,8,13-14H2,1H3;/q-1;+1
Klucz InChI
OXCMYAYHXIHQOA-UHFFFAOYSA-N
informacje o genach
human ... AGTR1(185)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
- Pharmaceutical formulations using reversed-phase high-performance liquid chromatography and UV-spectrophotometric techniques.
- Rat plasma using liquid chromatography–tandem mass spectrometry (LC–MS/MS) technique.
Działania biochem./fizjol.
Polecane produkty
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej