Kluczowe dokumenty
42147
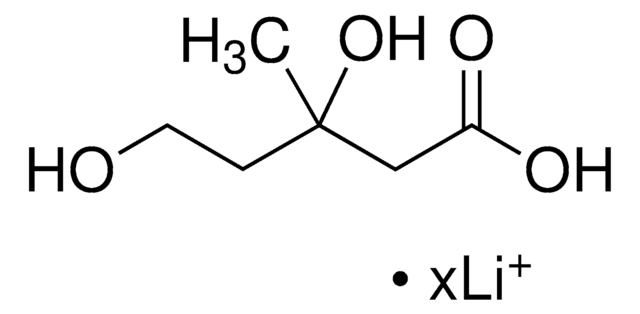
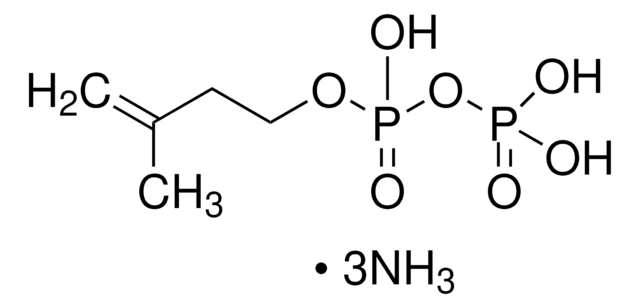
(RS)-Mevalonic acid lithium salt
analytical standard
Synonim(y):
(±)-MVA-Li, rac.-MVA-Li, Lithium (±)-3,5-dihydroxy-3-methyl-pentanoate, Lithium (±)-3,5-dihydroxy-3-methyl-valerate
About This Item
Polecane produkty
klasa czystości
analytical standard
Poziom jakości
Próba
≥96.0% (GC)
okres trwałości
limited shelf life, expiry date on the label
metody
HPLC: suitable
gas chromatography (GC): suitable
Format
neat
ciąg SMILES
OC(CCO)(CC(=O)O)C
InChI
1S/C6H12O4/c1-6(10,2-3-7)4-5(8)9/h7,10H,2-4H2,1H3,(H,8,9)
Klucz InChI
KJTLQQUUPVSXIM-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
Działania biochem./fizjol.
Opakowanie
Komentarz do analizy
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej