Wszystkie zdjęcia(1)
Kluczowe dokumenty
10110434001
Roche
Oksydaza ksantynowa (XOD)
from cow milk
Synonim(y):
XOD
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Polecane produkty
pochodzenie biologiczne
bovine milk
Poziom jakości
Formularz
suspension
aktywność właściwa
~1 units/mg protein (At 25 °C with xanthine as the substrate.)
opakowanie
pkg of 1 mL (20 U)
producent / nazwa handlowa
Roche
optymalne pH
8.5-9.0
Warunki transportu
wet ice
temp. przechowywania
2-8°C
Powiązane kategorie
Opis ogólny
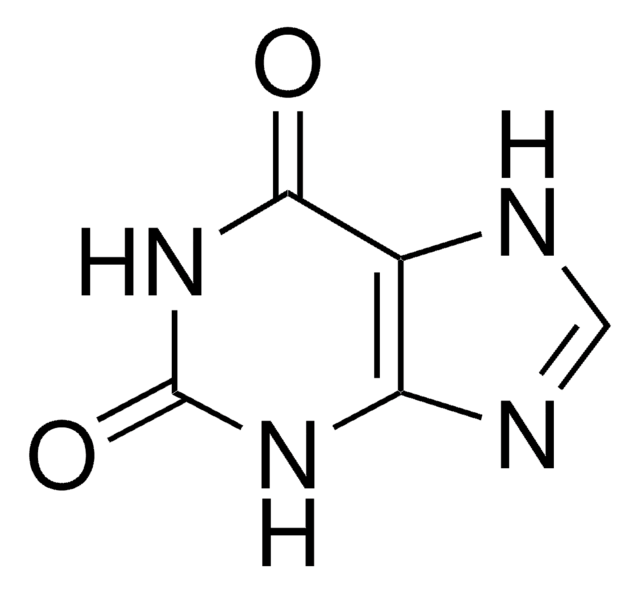
Xanthine Oxidase (XOD) is a metal flavoprotein. It has FAD, molybdenum and iron in the ratio 2:2:8.
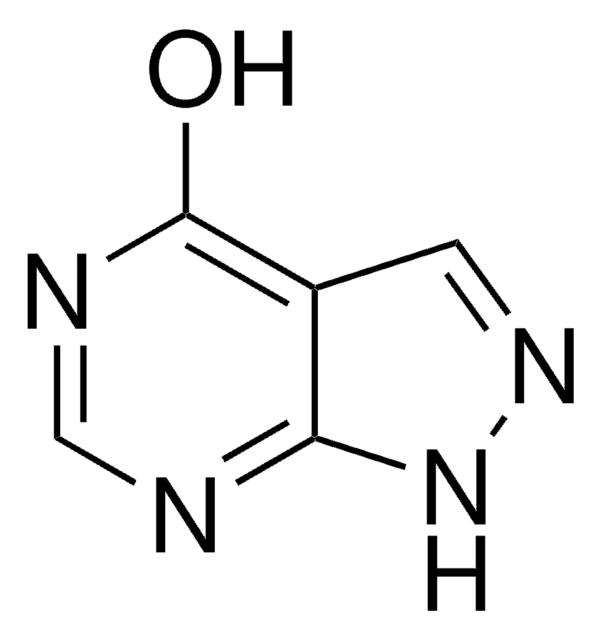
Xanthine:oxygen oxidoreductase
Zastosowanie
Xanthine Oxidase (XOD) has been used in the assessment of XOR-mediated NO production from NDHP and assessment of nitrite-derived NO in liver and purified XOR.
Xanthine Oxidase has been used to study tyrosine nitration.
Działania biochem./fizjol.
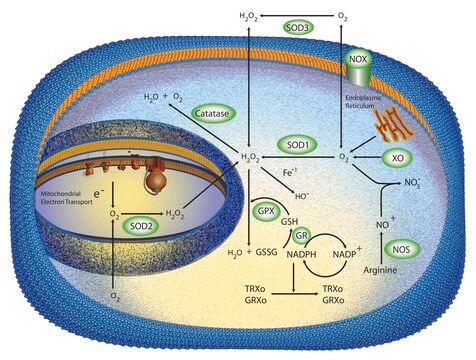
Xanthine Oxidase (XOD) exhibits a broad substrate specificity including aldehydes, purines and pteridines. Furthermore, this enzyme reduces oxygen to generate superoxide, hydrogen peroxide and reactive oxygen species (ROS). It also reduces nitrite to yield reactive nitrogen species (RNS), such as peroxynitrite and nitric oxide. Owing to its ability to generate RNS and ROS, XOD might play an important role as an antimicrobial agent in the neonatal gut, thereby complementing endogenous enzyme of the intestinal epithelium.
Jakość
Contaminants: <0.005% guanase, NP and uricase, each, <0.05% ADA, <0.05% alkaline phosphatase (4-nitrophenyl phosphate as the substrate)
Note: Chromatographically purified.
Note: Chromatographically purified.
Sekwencja
XOD is a dimer. Each subunit contains 1 atom of molybdenum, 2 iron-sulfur centers (non-heme iron, ferredoxin-type) and 1 molecule of FAD.
Definicja jednostki
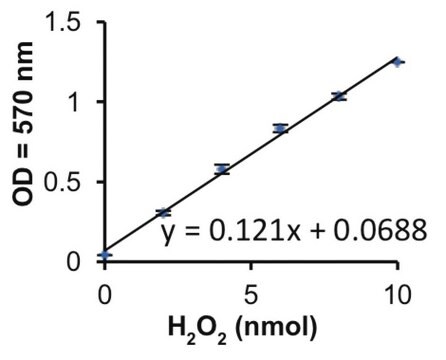
One unit (U) xanthine oxidase will produce 1 μmol of uric acid (E293nm = 12.2 mmol -1 x L x cm-1) from the oxidation of 1 μmol of xanthine in 1 min at 25 °C and pH8.5.
Postać fizyczna
Suspension in 3.2 M ammonium sulfate solution, 10 mM EDTA, pH approximately 8
Uwaga dotycząca przygotowania
Activator: O2
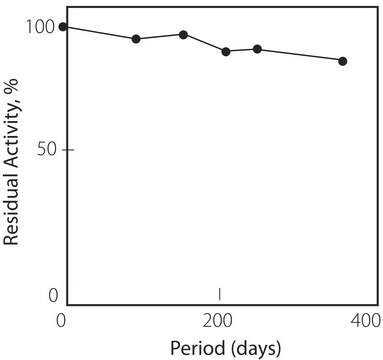
Stabilizers: The substance is stabilized by the addition of EDTA; salicylate is not added.
Stabilizers: The substance is stabilized by the addition of EDTA; salicylate is not added.
Inne uwagi
For life science research only. Not for use in diagnostic procedures.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
12 - Non Combustible Liquids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
does not flash
Temperatura zapłonu (°C)
does not flash
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
T Sawa et al.
The Journal of biological chemistry, 275(42), 32467-32474 (2000-07-25)
Peroxynitrite (ONOO(-)) is a potent nitrating and oxidizing agent that is formed by a rapid reaction of nitric oxide (NO) with superoxide anion (O(2)). It appears to be involved in the pathophysiology of many inflammatory and neurodegenerative diseases. It has
Methods of Enzymatic Analysis (2012)
The novel organic mononitrate NDHP attenuates hypertension and endothelial dysfunction in hypertensive rats.
Paulo LL, et al.
Redox Biology, 15, 182-191 (2018)
International Dairy Journal
R. Harrison.
Science, 16 (6), 546-554 (2006)
Synthesis and characterization of a novel organic nitrate NDHP: Role of xanthine oxidoreductase-mediated nitric oxide formation.
Zhuge Z, et al.
Redox Biology, 13, 163-169 (2017)
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej