MM2PLTVP1
Milliflex Oasis® Customer Validation Protocol in letter format
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Kod UNSPSC:
41116500
NACRES:
NB.79
Polecane produkty
Zastosowanie
bioburden testing
cosmetics
pharmaceutical
water monitoring
kompatybilność
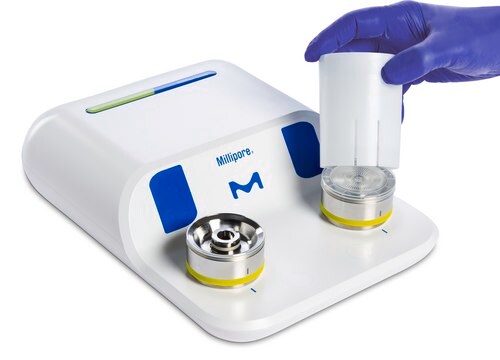
for use with MILLIFLEX®
Opis ogólny
Microbiological monitoring and testing in the pharmaceutical industry are highly regulated and a very complex field. In our long history of serving the pharmaceutical industry by pioneering and refining ground-breaking solutions, we have gained the regulatory and technical expertise to make compliance as simple as possible, and help save your valuable resources, by providing comprehensive range of professional and best-in-class services. Our Customer Validation Protocol makes validation faster, easier and ensures that your Milliflex Oasis® pump, accessories and related consumables do comply with the validated specifications. Our bioburden validation protocols follow international guidelines such as EP/USP and GMP.
With our best-in-class services you can:
With our best-in-class services you can:
- Optimize your QC lab workflow and ensure regulatory compliance
- Rely on comprehensive and ready-to-use validation packages
- Ensure the performance of your Milliflex Oasis® pump while reducing the risk of breakdown
Zastosowanie
Our validation protocols are based on our internal product qualification test methods. These extensive protocols will enable the QC/QA lab to quickly initiate the validation master plan and perform IQ, OQ and PQ (suitability of the test methodology) with ease.
Cechy i korzyści
Count on our comprehensive and ready-to-use validation protocols consisting of the following sections:
1. Validation Master Plan: Define structure, responsibilities for qualification
2. Installation Qualification (IQ)
4. Performance Qualification (PQ) Test Method suitability verification (microbiology validation procedures)
5. Final Report Summarizes all testing performed for final approval of validation
1. Validation Master Plan: Define structure, responsibilities for qualification
2. Installation Qualification (IQ)
- Verification and identification of with your Milliflex Oasis® pump, accessories and related consumables
- Verification of product′s utilities and operating environment requirements
- Equipment and personnel preparation
4. Performance Qualification (PQ) Test Method suitability verification (microbiology validation procedures)
5. Final Report Summarizes all testing performed for final approval of validation
Informacje prawne
MILLIFLEX is a registered trademark of Merck KGaA, Darmstadt, Germany
MILLIFLEX OASIS is a registered trademark of Merck KGaA, Darmstadt, Germany
This page may contain text that has been machine translated.
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Produkty
System Milliflex Oasis® monitoruje obciążenie biologiczne w różnych próbkach, zwiększając wydajność laboratorium.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej