495604
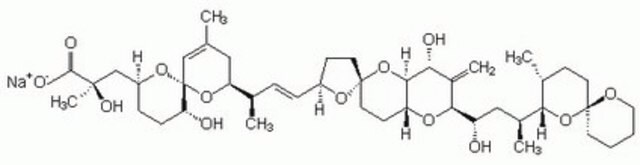
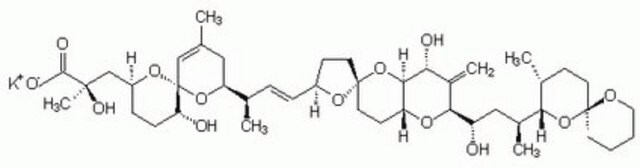
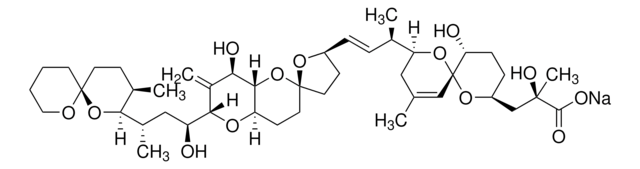
Okadaic acid
from Prorocentrum sp., ≥95% (HPLC), solid (glassy), protein phosphatase 1 inhibitor, Calbiochem
Synonim(y):
Okadaic Acid, Prorocentrum sp., OA
About This Item
Polecane produkty
product name
Okadaic Acid, Prorocentrum sp., Okadaic Acid, CAS 78111-17-8, is a highly potent inhibitor of protein phosphatase 1 (IC₅₀ = 10-15 nM) and 2A (IC₅₀ = 0.1 nM).
Poziom jakości
Próba
≥95% (HPLC)
Postać
solid (glassy)
producent / nazwa handlowa
Calbiochem®
warunki przechowywania
OK to freeze
protect from light
kolor
colorless
rozpuszczalność
DMF: soluble
chloroform: soluble
ethanol: soluble
methanol: soluble
Warunki transportu
ambient
temp. przechowywania
−20°C
InChI
1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37?,38+,39?,41-,42+,43-,44-/m1/s1
Klucz InChI
QNDVLZJODHBUFM-AAWJMCDUSA-N
Opis ogólny
Działania biochem./fizjol.
Protein phosphatase
Opakowanie
Ostrzeżenie
Rekonstytucja
Inne uwagi
Kiguchi, K., et al. 1994. Cell Growth Differentiation5, 995.
Ohaka, Y., et al. 1993. Biochem. Biophys. Res. Commun. 197, 916.
Gopalakrishna, R., et al. 1992. Biochem. Biophys. Res. Commun. 189, 950.
Kreienbuhl, P., et al. 1992. Blood80, 2911.
Nomura, M., et al. 1992. Biochemistry31, 11915.
Song, Q., et al. 1992. J. Cell Physiol.153, 550.
Tada, Y., et al. 1992. Immunopharmacol.24, 17.
Cohen, P., et al. 1990. Trends Biochem. Sci.15, 98.
Cohen, P. 1989. Annu. Rev. Biochem.58, 453.
Cohen, P., and Cohen, P.T. 1989. J. Biol. Chem.264, 21435.
Haystead, T.A., et al. 1989. Nature337, 78.
Informacje prawne
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Skin Irrit. 2
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej