440202
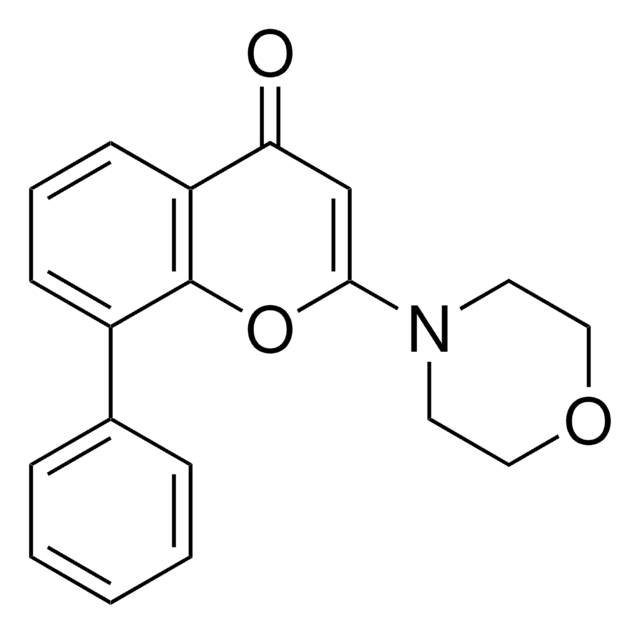
LY 294002
≥98% (HPLC), solid, PI3K inhibitor, Calbiochem®
Synonim(y):
2-(4-morfolinylo)-8-fenylo-4H-1-benzopiran-4-on, inhibitor BRD2 IV, inhibitor BRD3 III, inhibitor BRD4 IV
About This Item
Polecane produkty
Nazwa produktu
LY 294002, LY294002, CAS 154447-36-6, is a cell-permeable, potent, reversible, and specific inhibitor of PI 3-kinase ((IC₅₀ = 1.4 µM). Acts on the ATP-binding site.
Poziom jakości
Próba
≥98% (HPLC)
Formularz
solid
producent / nazwa handlowa
Calbiochem®
warunki przechowywania
OK to freeze
protect from light
kolor
off-white
rozpuszczalność
DMSO: 20 mg/mL
ethanol: soluble
Warunki transportu
ambient
temp. przechowywania
−20°C
ciąg SMILES
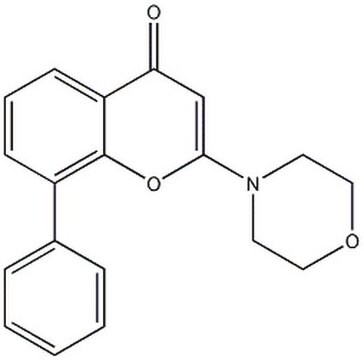
N4(CCOCC4)c1[o]c2c([c](c1)=O)cccc2c3ccccc3
InChI
1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2
Klucz InChI
CZQHHVNHHHRRDU-UHFFFAOYSA-N
Opis ogólny
Działania biochem./fizjol.
Phosphatidylinositol 3-kinase
Ostrzeżenie
Uwaga dotycząca przygotowania
Rekonstytucja
Inne uwagi
Bechard, M., and Dalton. S. 2009. Mol. Cell. Biol.29, 2092.
Lianguzova, M.S. et al. 2007. Cell Biol. Int.31, 330.
Baumann, P., and West, S.C. 1998. Proc. Natl. Acad. Sci. USA 95, 14066.
Cardone, M.H., et al. 1998. Science282, 1318.
Vlahos, C.J., et al. 1995. J. Immunol.154, 2413.
Yano, H., et al. 1995. Biochem. J.312, 145.
Vlahos, C.J., et al. 1994. J. Biol. Chem.269, 5241.
Selected Citations
Lee, J., et al. 2009. Cell Stem Cell5, 76.
Informacje prawne
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
487.4 °F
Temperatura zapłonu (°C)
253.0 °C
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej