Wszystkie zdjęcia(1)
Kluczowe dokumenty
S4921
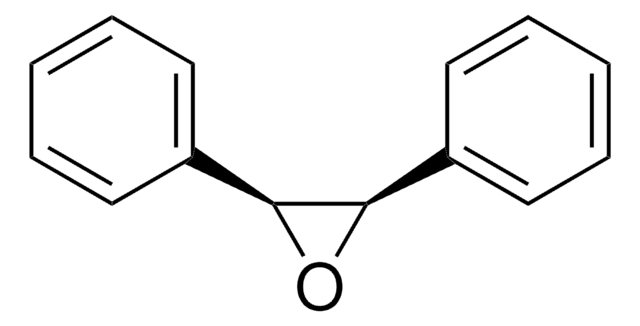
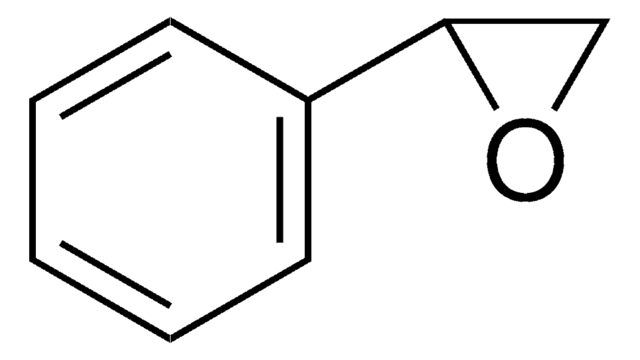
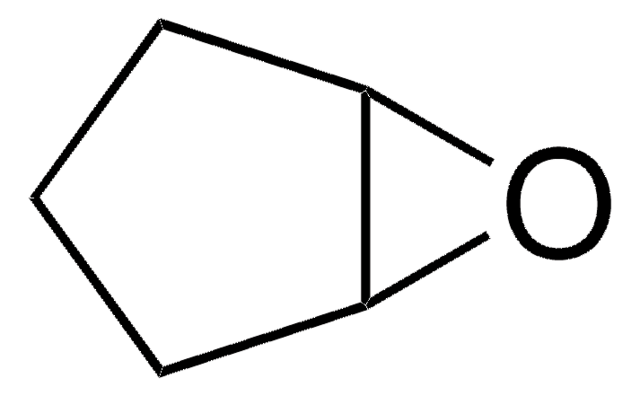
trans-Stilbene oxide
98%
Synonim(y):
trans-1,2-Diphenyloxirane
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C14H12O
Numer CAS:
Masa cząsteczkowa:
196.24
Beilstein:
82740
Numer WE:
Numer MDL:
Kod UNSPSC:
12352100
Identyfikator substancji w PubChem:
NACRES:
NA.22
Polecane produkty
Poziom jakości
Próba
98%
mp
65-67 °C (lit.)
ciąg SMILES
O1[C@@H]([C@H]1c2ccccc2)c3ccccc3
InChI
1S/C14H12O/c1-3-7-11(8-4-1)13-14(15-13)12-9-5-2-6-10-12/h1-10,13-14H/t13-,14-/m1/s1
Klucz InChI
ARCJQKUWGAZPFX-ZIAGYGMSSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Trans-stilbene oxide also known as trans-1,2-Diphenyloxirane, is often used in photochemistry, whereit can change its structure when exposed to light.Stilbene oxides can break apart when they are excited bylight, leading to the formation of carbonyl ylides. It is also commonlyused to produce trans-stilbene sulfides.
Zastosowanie
- Chiral Stationary Phases for Liquid Chromatography: Trans-stilbene oxide has been utilized in the fabrication of cellulose derivative-coated spherical covalent organic frameworks, serving as chiral stationary phases for high-performance liquid chromatographic enantioseparation, demonstrating its pivotal role in advanced analytical methodologies (Yan et al., 2022).
- Method Selection for Chiral High-Performance Liquid Chromatography: Its application extends to the utilization of hysteresis phenomena for chiral high-performance liquid chromatographic method selection in polar organic mode, enhancing the efficiency and specificity of pharmaceutical compound analysis (Horváth et al., 2020).
- Adsorption Properties for Enantioseparations: The effect of chiral selector loading on the adsorption properties of fully- and superficially-porous particles is crucial for high-efficient ultrafast enantioseparations, where trans-stilbene oxide derivatives play a significant role (Felletti et al., 2018).
- Catalysis in Alkene Epoxidation: Trans-stilbene oxide is involved in innovative catalysis research, specifically in the development of carbon nitride-supported Fe(2) cluster catalysts for alkene epoxidation, showcasing its utility in sustainable chemical synthesis (Tian et al., 2018).
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Neil Everall et al.
Applied spectroscopy, 64(1), 52-60 (2010-02-06)
Picosecond time-resolved transmission Raman data were acquired for 1 mm thick powder samples of trans-stilbene, and a Monte Carlo model was developed that can successfully model the laser and Raman pulse profiles. Photon migration broadened the incident (approximately 1 ps)
Joel Putnam et al.
Journal of chromatography. A, 1218(31), 5157-5165 (2011-07-06)
Acid/base mobile phase modifiers affect enantioseparations in ways that are not yet understood for the lack of systematic studies, which makes the scale-up of preparative separations difficult to predict. Shifts of the selectivity of certain pairs of enantiomers upon exposure
A L Slitt et al.
Drug metabolism and disposition: the biological fate of chemicals, 34(7), 1190-1197 (2006-04-20)
trans-Stilbene oxide (TSO) is a synthetic proestrogen that induces phase I and II drug-metabolizing enzymes in rat liver. The purpose of this study was to determine whether TSO also induces transporter expression in rat liver and whether gene induction in
V J Mayani et al.
Chirality, 21(2), 255-261 (2008-06-19)
Chromatographic behavior of nonracemic mixtures, viz., mandelic acid and stilbene oxide as analytes has been studied in detailed by enantiomer self-disproportionation on achiral ordered mesoporous material M41S and regular silica gel as stationary phases. Enantiomer self-disproportionation gave enhanced separation of
Li-Ming Yang et al.
Bioorganic & medicinal chemistry letters, 12(7), 1013-1015 (2002-03-23)
A new series of trans-stilbene benzenesulfonamide derivatives were designed and synthesized as potential antitumor agents. These new compounds were evaluated in the National Cancer Institute's 60 human tumor cell line in vitro screen. Compounds 9-13 were cytotoxic against several cell
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej