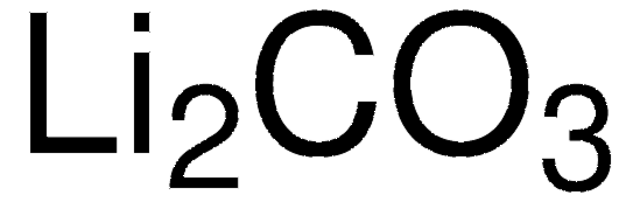Kluczowe dokumenty
930903
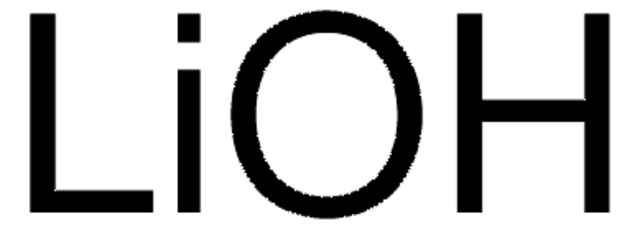
Lithium hydroxide monohydrate
battery grade, ≥99.9% trace metals basis
Synonim(y):
Lithine hydrate, Lithium hydroxide hydrate
About This Item
Polecane produkty
Poziom jakości
klasa czystości
battery grade
Próba
≥99.9% trace metals basis
Postać
powder
charakterystyka ekologicznej alternatywy
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
zanieczyszczenia
≤1000 ppm (trace metals analysis)
mp
423 °C
rozpuszczalność
H2O: soluble ((lit.))
ethanol: slightly soluble ((lit.))
methanol: soluble ((lit.))
ślady anionów
chloride (Cl-): ≤50 ppm
sulfate (SO42-): ≤50 ppm
Zastosowanie
battery manufacturing
kategoria ekologicznej alternatywy
ciąg SMILES
[Li+].O.[OH-]
InChI
1S/Li.2H2O/h;2*1H2/q+1;;/p-1
Klucz InChI
GLXDVVHUTZTUQK-UHFFFAOYSA-M
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Lithium hydroxide is produced in several ways. Most commonly, lithium carbonate is reacted with calcium hydroxide in a metathesis reaction. This directly yields lithium hydroxide hydrate, which is separated from the insoluble calcium carbonate byproduct and purified. Alternatively, when the source of lithium is spodumene ore, the ore can be converted to lithium hydroxide without first forming the carbonate. In the process, the lithium ore is treated with high-temperatures and sulfuric acid to form lithium sulfate; then the lithium sulfate is reacted with sodium hydroxide to form lithium hydroxide hydrate, which is purified.
Zastosowanie
Our battery grade lithium hydroxide monohydrate is well-suited for synthesis of nickel-rich metal oxides, like lithium nickel-manganese-aluminum oxide (NMA) and complex quaternary transition metal oxides like Zr-doped or Ti-doped nickel-manganese oxide.
Our lithium hydroxide monohydrate can also be used to synthesize lithium iron phosphates like LiFePO4 or lithium manganese oxides like Li2Mn2O4.
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Kod klasy składowania
8A - Combustible corrosive hazardous materials
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej