Kluczowe dokumenty
856061
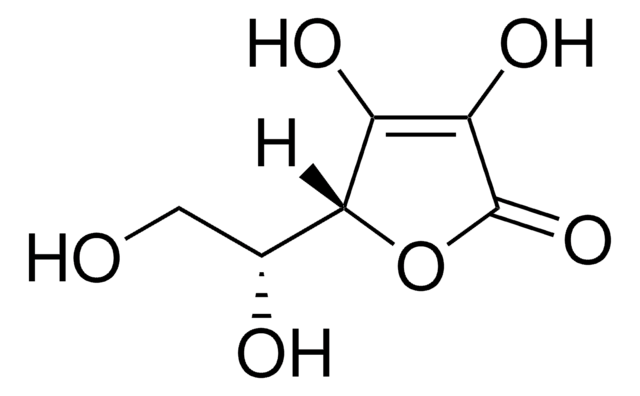
D-(−)-Isoascorbic acid
98%
Synonim(y):
D-erythro-Hex-2-enoic acid γ-lactone, D-Araboascorbic acid, Erythorbic acid, Glucosaccharonic acid, NSC 8117
About This Item
Polecane produkty
Poziom jakości
Próba
98%
Formularz
crystals
aktywność optyczna
[α]25/D −16.8°, c = 2 in H2O
mp
169-172 °C (dec.) (lit.)
ciąg SMILES
[H][C@@]1(OC(=O)C(O)=C1O)[C@H](O)CO
InChI
1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5-/m1/s1
Klucz InChI
CIWBSHSKHKDKBQ-DUZGATOHSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
- enantiopure aminotriol
- (3R, 4S)-4-hydroxylasiodiplodin and D-mycinose
- enantiomerically pure stereoisomers of α,β-dihydroxy-aldehydes or acids
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 2
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej