Key Documents
803367
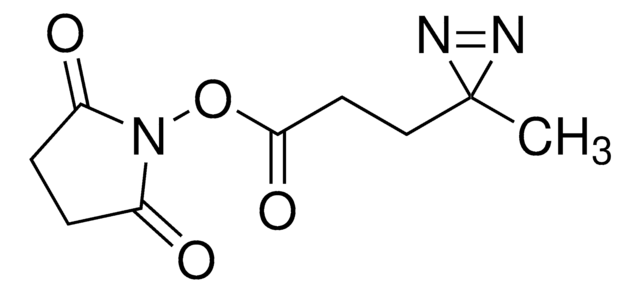
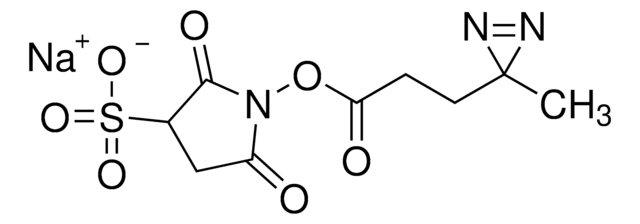
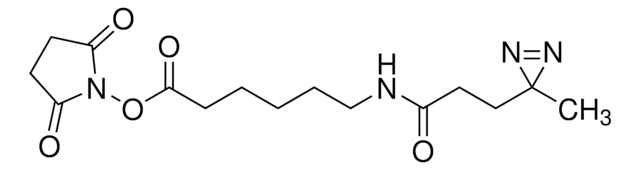
Sulfo-SDAD (Sulfo-NHS-SS-Diazirine) (sulfosuccinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithiopropionate]
About This Item
Polecane produkty
Próba
≥80%
Postać
powder
masa cząsteczkowa
490.51
przydatność reakcji
reagent type: cross-linking reagent
warunki przechowywania
desiccated
rozpuszczalność
water: soluble
Warunki transportu
ambient
temp. przechowywania
2-8°C
ciąg SMILES
CC1(N=N1)CCC(NCCSSCCC(ON2C(C(S([O-])(=O)=O)CC2=O)=O)=O)=O.[Na+]
InChI
1S/C14H20N4O8S3.Na/c1-14(16-17-14)4-2-10(19)15-5-7-28-27-6-3-12(21)26-18-11(20)8-9(13(18)22)29(23,24)25;/h9H,2-8H2,1H3,(H,15,19)(H,23,24,25);/q;+1/p-1
Klucz InChI
BTBHSSBEJHAIAF-UHFFFAOYSA-M
Opis ogólny
Cechy i korzyści
- Water soluble—solubility in aqueous solutions improved by a sulfonate group
- Heterobifunctional—NHS ester group reacts with primary amines at pH 7 to 9 to form covalent amide bonds; diazirine (azipentanoate) group reacts efficiently with any amino acid side chain or peptide backbone upon activation with long-wave UV light (330-370 nm)
- Controllable—two-step chemical crosslinking is activated using common laboratory UV lamps
- Easy to use—these crosslinkers are photo-stable under typical laboratory lighting conditions so there is no need to perform experiments in the dark
- Better than aryl azides—the diazirine photoreactive group has better photostability in normal light than phenyl azide groups of traditional photoreactive crosslinkers, yet the diazirine group is more efficiently activated by long-wave UV light
Przestroga
produkt powiązany
Kod klasy składowania
13 - Non Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej