Wszystkie zdjęcia(1)
Key Documents
426016
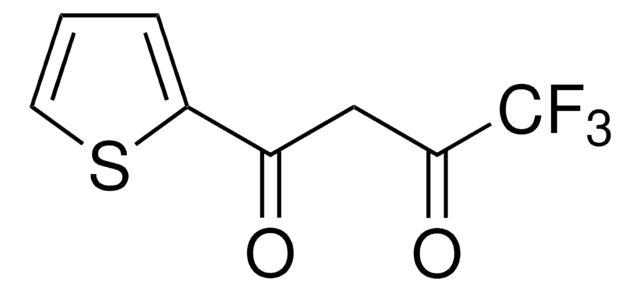
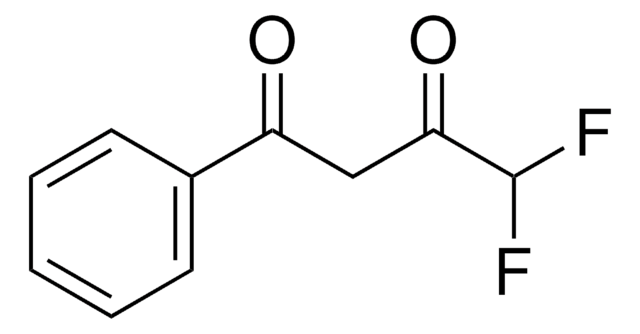
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione
99%
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C8H5F3O3
Numer CAS:
Masa cząsteczkowa:
206.12
Numer WE:
Numer MDL:
Kod UNSPSC:
12352100
Identyfikator substancji w PubChem:
NACRES:
NA.22
Polecane produkty
Poziom jakości
Próba
99%
Postać
liquid
współczynnik refrakcji
n20/D 1.528 (lit.)
tw
203 °C (lit.)
mp
19-21 °C (lit.)
gęstość
1.391 g/mL at 25 °C (lit.)
temp. przechowywania
2-8°C
ciąg SMILES
FC(F)(F)C(=O)CC(=O)c1ccco1
InChI
1S/C8H5F3O3/c9-8(10,11)7(13)4-5(12)6-2-1-3-14-6/h1-3H,4H2
Klucz InChI
OWLPCALGCHDBCN-UHFFFAOYSA-N
Opis ogólny
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione (furoyltrifluoroacetone, FTFA) is a β-diketone. Its cytotoxic activity against human cultured tumor and normal cells has been evaluated. Reports suggest that 4,4,4-trifluoro-1-(2-furyl)-1,3-butanedione partially inhibits the oxidation of ferrocyanide in ETP (electron transport particles) isolated from beef heart mitochondria. Its reaction with N,N,N′,N′-tetramethylalkyl diamines to form ionic adducts has been investigated. The conformational analysis of the enol and keto form of FTFA has been reported.
Zastosowanie
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione (tfa) may be used in the following studies:
- As capping ligand in the synthesis of [Eu(tfa)3]2bpm complexes (bpm=2,2′-bipyrimidine).
- As reagent in the multistep synthesis of [13CD2]benzylamine.
- As reagent in the synthesis of 3-trifluoromethyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide derivatives by reacting with corresponding benzofurazan oxides.
- In the efficient syntheses of perfluoroalkyl substituted azoles.
- Synthesis of 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives.
This page may contain text that has been machine translated.
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
188.6 °F - closed cup
Temperatura zapłonu (°C)
87 °C - closed cup
Środki ochrony indywidualnej
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
X-ray structure and temperature dependent luminescent properties of two bimetallic europium complexes.
Swavey S, et al.
Polyhedron, 27(3), 1061-1069 (2008)
An efficient entry to perfluoroalkyl substituted azoles starting from β-perfluoroalkyl-β-dicarbonyl compounds.
Bravo P, et al.
Tetrahedron, 50(29), 8827-8836 (1994)
Kensuke Nakano et al.
Anticancer research, 24(2B), 711-717 (2004-05-27)
A variety of beta-diketones were evaluated for their cytotoxic profiles against oral human normal and tumor cells. Among 22 compounds (BD1-22) tested, the cytotoxicity of 3-formylchromone (BD17) (CC50=7.8 microg/mL) against human oral squamous cell carcinoma (HSC-2) cells was higher than
Conformation, structure, intramolecular hydrogen bonding, and vibrational assignment of 4,4, 4-trifluoro-1-(2-furyl)-1,3-butanedione.
Tayyari SF, et al.
Journal of Molecular Structure, 8882(1), 153-167 (2008)
A Mutlib et al.
Drug metabolism and disposition: the biological fate of chemicals, 29(10), 1296-1306 (2001-09-19)
The role of gamma-glutamyltranspeptidase (GGT) in transferring glutamate from endogenous glutathione (GSH) to the benzylamine moiety of a compound, such as 1-[3-(aminomethyl)phenyl]-N-[3-fluoro-2'-(methylsulfonyl)-[1,1'-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide (DPC 423), is described. Studies were performed with structurally related analogs of DPC 423 to demonstrate that this
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej