추천 제품
분석
≥90% (LC/MS-ELSD)
형태
solid
응용 분야
metabolomics
vitamins, nutraceuticals, and natural products
저장 온도
−20°C
SMILES string
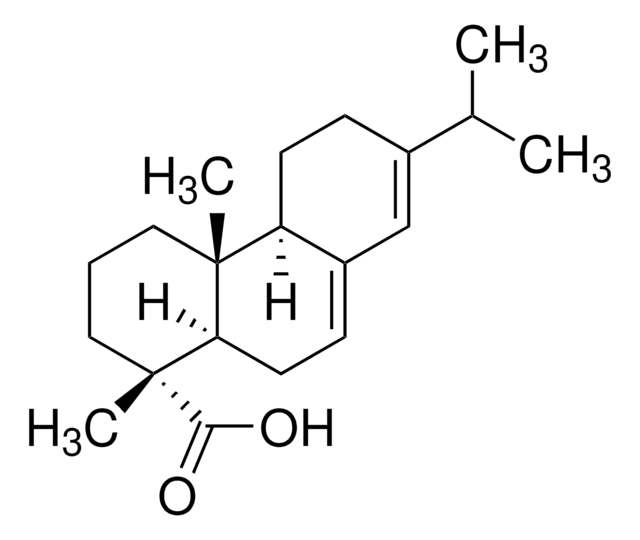
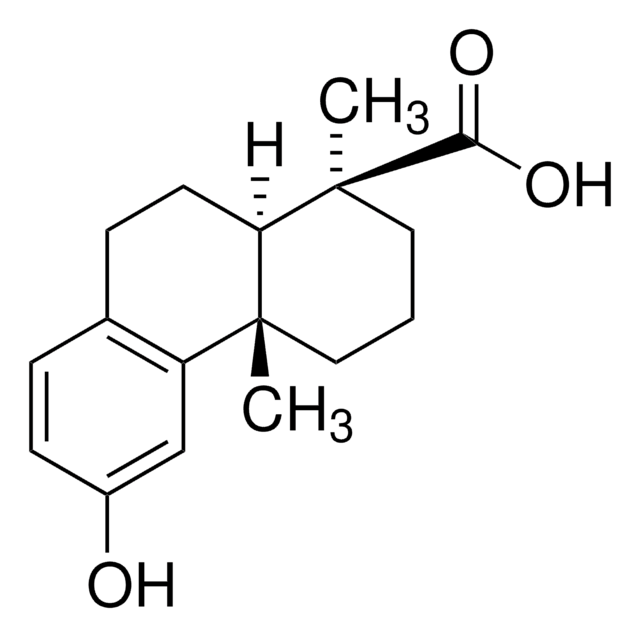
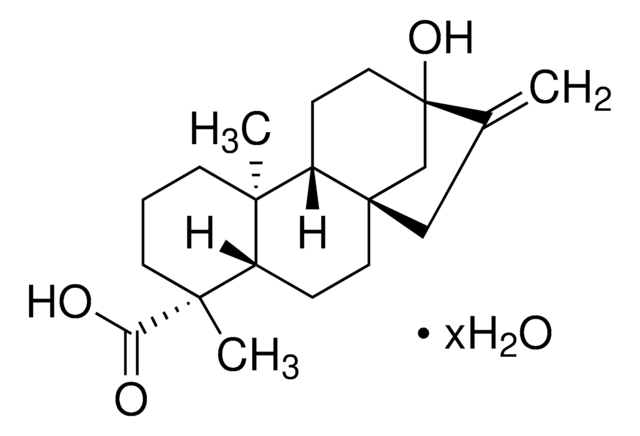
CC(C)c1ccc2c(CC[C@H]3[C@@](C)(CCC[C@]23C)C(O)=O)c1
InChI
1S/C20H28O2/c1-13(2)14-6-8-16-15(12-14)7-9-17-19(16,3)10-5-11-20(17,4)18(21)22/h6,8,12-13,17H,5,7,9-11H2,1-4H3,(H,21,22)/t17-,19-,20-/m1/s1
InChI key
NFWKVWVWBFBAOV-MISYRCLQSA-N
일반 설명
Dehydroabietic acid (DHA or DAA) is a bioactive phytochemical, diterpenoid found in various Pinus species. It is a resin acid and a derivative of abietic acid (AA).
애플리케이션
Dehydroabietic acid has been used as a reference standard:
- to study the aging process of Pinus resins using Fourier-transform infrared spectroscopy (FTIR)
- to study the composition changes in Pinus genus with aging using Raman spectroscopy complemented with infrared spectroscopy
- to estimate resistance against biotic stress as proxy in chemical defenses in Pinus halepensis
생화학적/생리학적 작용
Dehydroabietic acid (DHA) exerts various biological activities such as anti-cancer, anti-aging, antimicrobial, antiulcer, gastroprotective, and cytotoxic activities. It is a potent anti-inflammatory agent and a dual activator of peroxisome proliferator-activated receptors alpha and gamma (PPAR α/γ). DHA has an anti-aging effect and a sirtuin 1 (SIRT1) activating compound. It has antibacterial properties against multidrug-resistant strains. dehydroabietic acid and its derivatives have gastroprotective and cytotoxic effects.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Yong-Mei Cui et al.
Bioorganic & medicinal chemistry letters, 18(19), 5201-5205 (2008-09-16)
A series of dehydroabietic acid (DHAA, 2) derivatives was synthesized and evaluated as BK channel openers in an assay system of CHO-K1 cells expressing hBKalpha channels. Systematic modifications of the peripheral functionality of ring C of DHAA showed that the
Teris A van Beek et al.
Journal of natural products, 70(2), 154-159 (2007-02-24)
Dehydroabietic acid (DHA) (1) is one of the main compounds in Scots pine wood responsible for aquatic and microbial toxicity. The degradation of 1 by Trametes versicolor and Phlebiopsis gigantea in liquid stationary cultures was followed by HPLC-DAD-ELSD. Both fungi
Nor Fadhilah Kamaruzzaman et al.
Materials (Basel, Switzerland), 11(9) (2018-09-16)
Infectious disease caused by pathogenic bacteria continues to be the primary challenge to humanity. Antimicrobial resistance and microbial biofilm formation in part, lead to treatment failures. The formation of biofilms by nosocomial pathogens such as Staphylococcus aureus (S. aureus), Pseudomonas
Xianxing Jiang et al.
Organic letters, 11(1), 153-156 (2008-12-11)
A new class of dehydroabietic amine-substituted primary amine-thiourea bifunctional catalysts were designed and synthesized. The doubly stereocontrolled organocatalytic conjugate addition of a variety of heterocycles-bearing ketones to nitroalkenes was investigated for the first time, affording (S)- or (R)-gamma-nitro heteroaromatic ketones
Beatriz Sepúlveda et al.
Pharmacological research, 52(5), 429-437 (2005-08-30)
Dehydroabietic acid derivatives have been reported to display antisecretory and antipepsin effect in animal models. Some 19 dehydroabietic acid diterpenes were prepared and assessed for gastroprotective activity in the HCl/EtOH-induced gastric lesions in mice as well as for cytotoxicity in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.