119792
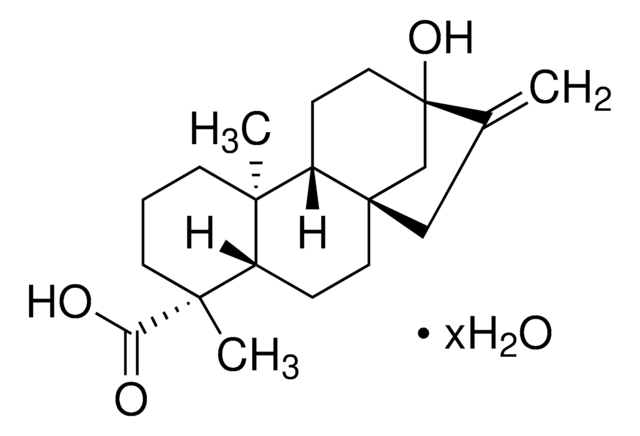
Podocarpic acid
98%
동의어(들):
(+)-Podocarpic acid, (1S)-1,2,3,4,4a,9,10,10a-Octahydro-6-hydroxy-1,4a-dimethyl-1-phenanthrenecarboxylic acid, (1S,4aS,10aR)-1,2,3,4,4a,9,10,10a-Octahydro-6-hydroxy-1,4a-dimethyl-1-phenanthrenecarboxylic acid, Podocarpic acid (resin acid)
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C17H22O3
CAS Number:
Molecular Weight:
274.35
EC Number:
MDL number:
UNSPSC 코드:
12352002
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
형태
solid
광학 활성
[α]20/D +133°, c = 4 in ethanol
mp
193-196 °C (lit.)
SMILES string
[H][C@@]12CCc3ccc(O)cc3[C@@]1(C)CCC[C@]2(C)C(O)=O
InChI
1S/C17H22O3/c1-16-8-3-9-17(2,15(19)20)14(16)7-5-11-4-6-12(18)10-13(11)16/h4,6,10,14,18H,3,5,7-9H2,1-2H3,(H,19,20)/t14-,16-,17+/m1/s1
InChI key
VJILEYKNALCDDV-OIISXLGYSA-N
유전자 정보
human ... TNF(7124)
애플리케이션
- (+)-Podocarpic acid as chiral template in the synthesis of aphidicolane, stemodane and stemarane diterpenoids: This article reviews the use of (+)-podocarpic acid in the synthesis of various diterpenoids, showcasing its utility in complex organic syntheses (La Bella et al., 2016).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
E J Parish et al.
Journal of pharmaceutical sciences, 73(5), 694-696 (1984-05-01)
As a class, octahydrophenanthrene lactones, podolactones , and related podocarpic acid derivatives have been reported to possess a wide variety of biological activities, including antileukemic activity, inhibition of plant cell growth, and hormonal and anti-inflammatory properties. In the present study
W He et al.
Bioorganic & medicinal chemistry letters, 9(3), 469-474 (1999-03-26)
Podocarpic acid derivatives as cytokine (IL-1beta) release inhibitors are discussed.
Hany Nashaat Baraka
Planta medica, 76(8), 815-817 (2010-01-15)
Podocarpic acid was metabolized by Mucor ramannianus ATCC 9628, and Beauveria bassiana ATCC 7159 to afford two new metabolites, 2 alpha-hydroxy podocarpic acid and 11-hydroxy podocarpic acid, along with the known metabolite 13-hydroxy podocarbic acid. The identity of these metabolites
Toxic effects of some conifer resin acids and tea tree oil on human epithelial and fibroblast cells.
T A Söderberg et al.
Toxicology, 107(2), 99-109 (1996-02-22)
The present study was undertaken to assess and compare the in vitro cytotoxic effects of three resin acid analogues: dehydrobietic acid, podocarpic acid, O-methylpodocarpic acid; an essential oil from Australia (tea tree oil); and tapped oleoresin from Thailand, on human
Design, structure activity relationships and X-Ray co-crystallography of non-steroidal LXR agonists.
D J Bennett et al.
Current medicinal chemistry, 15(2), 195-209 (2008-01-29)
The Liver X Receptor (LXR) alpha and beta isoforms are members of the type II nuclear receptor family which function as a heterodimer with the Retinoid X Receptor (RXR). Upon agonist binding, the formation of the LXR/RXR heterodimer takes place
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.