357995
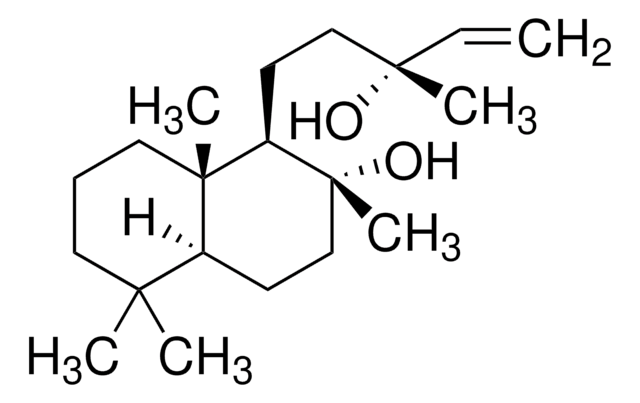
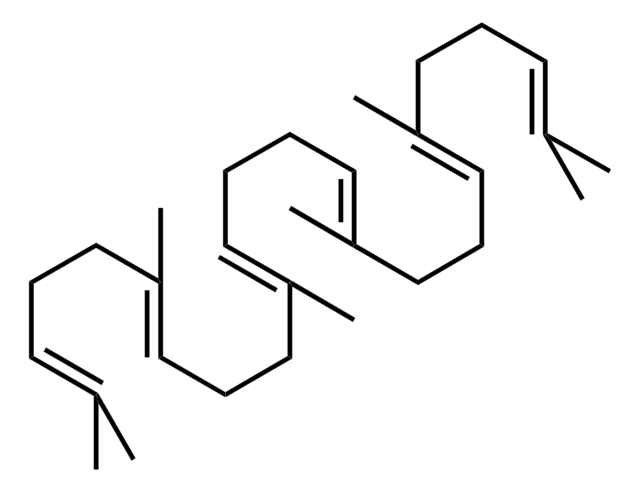
Sclareol
98%
동의어(들):
Labd-14-ene-8,13-diol, (1R,2R,8aS)-Decahydro-1-(3-hydroxy-3-methyl-4-pentenyl)-2,5,5,8a-tetramethyl-2-naphthol
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C20H36O2
CAS Number:
Molecular Weight:
308.50
Beilstein:
2054148
EC Number:
MDL number:
UNSPSC 코드:
12352002
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
광학 활성
[α]25/D −13°, c = 4 in carbon tetrachloride
bp
218-220 °C/19 mmHg (lit.)
mp
95-100 °C (lit.)
SMILES string
CC1(C)CCC[C@@]2(C)[C@H]1CC[C@@](C)(O)[C@@H]2CC[C@@](C)(O)C=C
InChI
1S/C20H36O2/c1-7-18(4,21)13-9-16-19(5)12-8-11-17(2,3)15(19)10-14-20(16,6)22/h7,15-16,21-22H,1,8-14H2,2-6H3/t15-,16+,18-,19-,20+/m0/s1
InChI key
XVULBTBTFGYVRC-HHUCQEJWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Sclareol, a diterpene compound with a labdane skeleton, is mainly used as a raw material in the fragrance industry. It shows potent cytotoxic and cytostatic effect in human leukemic cell lines.
애플리케이션
Sclareol may be used as a starting material in the synthesis of ambergris fragrance chemicals such as ambraoxide, ambrox, methylambraoxide, ambracetal, ambraketal and epiambraketal. It may also be used in the synthesis of (+)-galanolactone, (-)-8-epi-galanolactone and (+)-labdienedial.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
The effect of sclareol on growth and cell cycle progression of human leukemic cell lines.
Dimas K, et al.
Leukemia Research, 23(3), 217-234 (1999)
Synthesis of ambergris fragrance chemicals from sclareol, involving palladium catalysed key steps.
Coste-Maniere IC, et al.
Tetrahedron Letters, 29(9), 1017-1020 (1988)
Conversion of sclareol into (+)-galanolactone and (+)-labdienedial.
Jung M, et al.
Tetrahedron Letters, 38(16), 2871-2874 (1997)
Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture.
Caniard A, et al.
BMC plant biology, 12(1), 119-119 (2012)
A short efficient synthesis of ambraketal (four steps) and epiambraketal (five steps) from Sclareol.
Martres P, et al.
Tetrahedron Letters, 35(1), 97-98 (1994)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.