G1549
PNGase F from Elizabethkingia meningoseptica
ready-to-use solution, recombinant, expressed in E. coli
동의어(들):
PNGase F from Elizabethkingia meningoseptica, N-Glycosidase F, PNGase F from Chryseobacterium meningosepticum, PNGase F from Flavobacterium meningosepticum, Peptide N-glycosidase
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
추천 제품
재조합
expressed in E. coli
Quality Level
결합
(N-linked)
Grade
Proteomics Grade
형태
ready-to-use solution
특이 활성도
≥1000 U/mg
유통기한
≥1 yr at -20 °C
분자량
~36 kDa
배송 상태
wet ice
저장 온도
−20°C
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Recombinant PNGase F has been purified by affinity chromatography and dialyzed into a 50% glycerol solution with 10 mM potassium phoosphate pH 7.5 to produce a stable product. The product contains low levels of buffer salts. This highly purified material can be used for preparative deglycosylation or for analytical applications in gel, in solution, or on blot membranes. The enzyme can be removed from preparative operations by utilizing its C-terminal 6x histidine fusion tag. PNGase F from Elizabethkingia meningoseptica has been used in deglycosylation assay in human plasma samples and in deglycosylation of chondroitin sulfate proteoglycan.
Used to deglycosylate protein.
생화학적/생리학적 작용
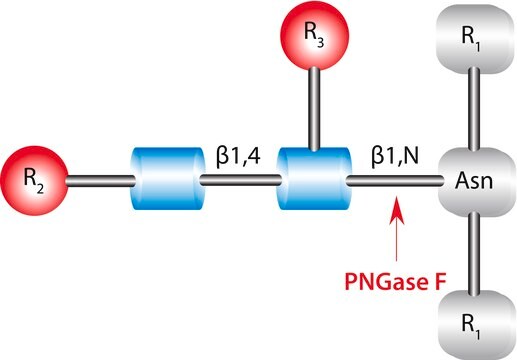
PNGase F from Elizabethkingia meningoseptica has glycan-binding catalytic domain and a bowl-like domain at the N-terminus. It cleaves an entire glycan from a glycoprotein provided the glycosylated asparagine moiety is substituted on its amino and carboxyl terminus with a polypeptide chain. It is cost-effectively produced on a large scale in prokaryotic hosts and requires divalent zinc ions for its enzymatic activity.
Cleaves an entire glycan from a glycoprotein provided the glycosylated asparagine moiety is substituted on its amino and carboxyl terminus with a polypeptide chain.
단위 정의
One unit will catalyze the release of N-linked oligosaccharides from 1 nanomole of denatured ribonuclease B in one minute at 37°C at pH 7.5 monitored by SDS-PAGE. One Sigma unit of PNGase F activity is equal to 1 IUB milliunit.
물리적 형태
Supplied as 300 Units/mL enzyme in 50% (v/v) glycerol and 50% (v/v) 20 mM Potassium Phosphate, pH 7.5.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
N-glycosylation of apolipoprotein A1 in cardiovascular diseases
Majek P, et al.
Translational Research, 165(2), 360-362 (2015)
Characterization and analysis of extracellular matrix in malignant brain tumors and their cellular derivatives
Extracellular Matrix, 113-138 (2015)
Discovery and characterization of a novel extremely acidic bacterial N-glycanase with combined advantages of PNGase F and A
Wang T, et al.
Bioscience Reports, 34(6), e00149-e00149 (2014)
Identification and characterization of a novel prokaryotic peptide: N-glycosidase from Elizabethkingia meningoseptica
Sun G, et al.
The Journal of Biological Chemistry, jbc-M114 (2015)
T H Plummer et al.
The Journal of biological chemistry, 259(17), 10700-10704 (1984-09-10)
Endo-beta-N-acetylglucosaminidase F preparations from Flavobacterium meningosepticum have been found to contain peptide:N-glycosidase activity. Only the second activity, designated as peptide:N-glycosidase F, readily cleaves the beta-aspartylglycosylamine linkage of a fetuin triantennary complex glycopeptide, as shown by the isolation of the corresponding
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.