P9120
PNGase F from Elizabethkingia meningoseptica
recombinant, expressed in E. coli, set of 100 units nanomolar unit
동의어(들):
N-Glycanase®, N-Glycosidase F, PNGase F from Chryseobacterium meningosepticum, PNGase F from Flavobacterium meningosepticum, Peptide N-glycosidase
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
CAS Number:
MDL number:
UNSPSC 코드:
12352204
NACRES:
NA.54
추천 제품
애플리케이션
PNGase F from Elizabethkingia meningoseptica has been used in deglycosylation
- of recombinant soybean agglutinin (rSBA) in Nicotiana benthamiana (NbrSBA) and Solanum tuberosum (StrSBA)
- of frontal cortical lysate to verify the glycosylation profile of β-secretase (BACE proteins)
- of cell lysate for evaluating the siRNA silencing of cellular prion protein (PrPc) post transfection
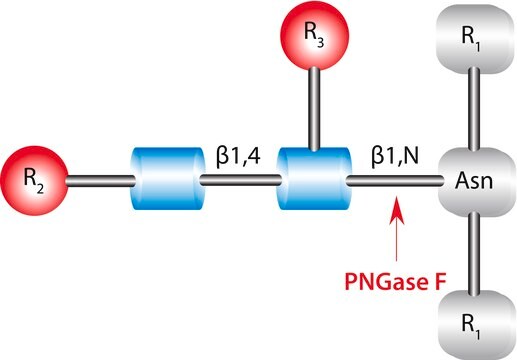
PNGase F is a glycosylasparaginase used to deglycosylate proteins. It is widely used in structure-function studies of glycoproteins.
Used to deglycosylate protein.
생화학적/생리학적 작용
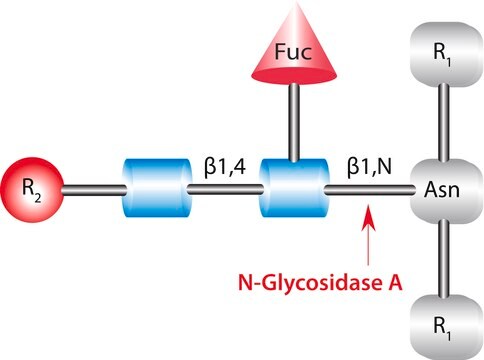
PNGase F cleaves an entire glycan from a glycoprotein provided the glycosylated asparagine moiety is substituted on its amino and carboxyl terminus with a polypeptide chain. It deaminates the asparagine to aspartic acid, but leaves the oligosaccharide intact. PNGase F will not remove oligosaccharides containing α(1-3)-linked core fucose, commonly found in plant glycoproteins. A tripeptide with the oligosaccharide-linked asparagine as the central residue is the minimal substrate for PNGase F.
Cleaves an entire glycan from a glycoprotein provided the glycosylated asparagine moiety is substituted on its amino and carboxyl terminus with a polypeptide chain.
포장
Each set includes enzyme, two formulations of 5× reaction buffer (for routine and mass spectrometry downstream analysis), detergent and denaturation solutions
단위 정의
One unit will catalyze the release of N-linked oligosaccharides from 1 micromole of denatured ribonuclease B in one minute at 37°C at pH 7.5 monitored by SDS-PAGE. One Sigma unit of PNGase F activity is equal to 1 IUB milliunit.
법적 정보
N-Glycanase is a registered trademark of Agilent Technologies Inc
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Repr. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
G E Norris et al.
Structure (London, England : 1993), 2(11), 1049-1059 (1994-11-15)
Peptide:N-glycosidase F (PNGase F) is an enzyme that catalyzes the complete removal of N-linked oligosaccharide chains from glycoproteins. Often called an endoglycosidase, it is more correctly termed an amidase or glycosylasparaginase as cleavage is at the asparagine-sugar amide linkage. The
Mitochondrial respiratory inhibition and oxidative stress elevate beta-secretase (BACE1) proteins and activity in vivo in the rat retina
Xiong K, et al.
Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale, 181(3), 435-446 (2007)
PrPc activation induces neurite outgrowth and differentiation in PC12 cells: role for caveolin-1 in the signal transduction pathway
Pantera B, et al.
Journal of Neurochemistry, 110(1), 194-207 (2009)
Reynald Tremblay et al.
Transgenic research, 20(2), 345-356 (2010-06-19)
Soybean agglutinin (SBA) is a specific N-acetylgalactosamine-binding plant lectin that can agglutinate a wide variety of cells. SBA has great potential for medical and biotechnology-focused applications, including screening and treatment of breast cancer, isolation of fetal cells from maternal blood
Amelie Croset et al.
Journal of biotechnology, 161(3), 336-348 (2012-07-21)
Glycosylation is one of the most common posttranslational modifications of proteins. It has important roles for protein structure, stability and functions. In vivo the glycostructures influence pharmacokinetics and immunogenecity. It is well known that significant differences in glycosylation and glycostructures
문서
N-Linked Glycan Strategies; Sigma-Aldrich.com
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.