모든 사진(1)
About This Item
실험식(Hill 표기법):
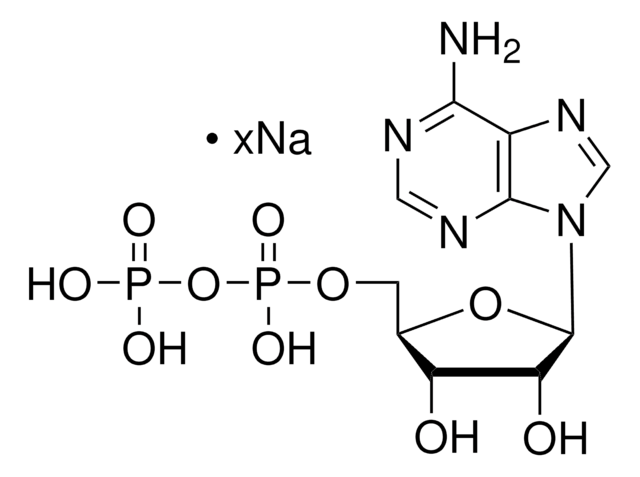
C10H12Li2N5O6PS
CAS Number:
Molecular Weight:
375.15
MDL number:
UNSPSC 코드:
41106305
PubChem Substance ID:
NACRES:
NA.51
추천 제품
Quality Level
분석
≥98%
형태
powder
저장 온도
−20°C
SMILES string
[Li+].[Li]SP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C10H14N5O6PS.2Li/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(21-10)1-20-22(18,19)23;;/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,23);;/q;2*+1/p-2/t4-,6-,7-,10-;;/m1../s1
InChI key
FZXPFDFAEUBVHA-IDIVVRGQSA-L
관련 카테고리
애플리케이션
Adenosine 5′′-O-thiomonophosphate (5′-AMPS) is a 5′-O-thiophosphate which along with adenosine 5′-O-(2-thiodiphosphate) and adenosine 5′-O-(3-thiotriphosphate) can act as inhibitors of phosphohydrolases. AMPS is a substrate for smooth muscle endothelial ecto-5′-nucleotidase. 5′APMS is a stimulator of 5-lipoxygenase activity of rat polymorphonuclear (PMN) leukocytes. Histidine triad nucleotide-binding protein 1 (HINT-1) phosphoramidase transforms nucleoside 5′-O-phosphorothioates such as 5′-AMPS to nucleoside 5′-O-phosphates.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - STOT SE 2
표적 기관
Eyes,Central nervous system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
J D Pearson et al.
The Biochemical journal, 230(2), 503-507 (1985-09-01)
We compared the properties of the ectonucleotidases (nucleoside triphosphatase, EC 3.6.1.15; nucleoside diphosphatase, EC 3.6.1.6; 5'-nucleotidase, EC 3.1.3.5) in intact pig aortic smooth-muscle cells in culture with the properties that we previously investigated for ectonucleotidases of aortic endothelial cells [Cusack
D Denis et al.
Archives of biochemistry and biophysics, 273(2), 592-596 (1989-09-01)
The stable nucleotide analog guanosine 5'-O-(3-thiotriphosphate) (GTP gamma S) was found to be a very potent activator of 5-lipoxygenase in cell-free preparations from rat polymorphonuclear (PMN) leukocytes, causing a 10-fold stimulation of arachidonic acid oxidation at concentrations as low as
Magdalena Ozga et al.
The Journal of biological chemistry, 285(52), 40809-40818 (2010-10-14)
Nucleoside 5'-O-phosphorothioates are formed in vivo as primary products of hydrolysis of oligo(nucleoside phosphorothioate)s (PS-oligos) that are applied as antisense therapeutic molecules. The biodistribution of PS-oligos and their pharmacokinetics have been widely reported, but little is known about their subsequent
D J Montague et al.
European journal of biochemistry, 139(3), 529-534 (1984-03-15)
Adenosine diphosphatase (ADPase) activity and ATPase activity were assayed in rat liver mitochondria and outer mitochondrial membrane preparations with [beta-32P]ADP and [gamma-32P]ATP as substrates. Inhibition studies were performed with the mitochondrial ATPase inhibitor oligomycin and the adenine nucleotide transport inhibitor
Adenosine 5'-phosphorothioate. A nucleotide analog that is a substrate, competitive inhibitor, or regulator of some enzymes that interact with adenosine 5'-phosphate.
A W Murray et al.
Biochemistry, 7(11), 4023-4029 (1968-11-01)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

