88185
Tetrazole solution
suitable for DNA synthesis, filtered through a 1 μm filter, ~0.45 M in acetonitrile
동의어(들):
1H-Tetrazole
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
CH2N4
CAS Number:
Molecular Weight:
70.05
Beilstein:
105799
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.21
bp:
84 °C (lit.)
추천 제품
양식
liquid
Quality Level
품질
filtered through a 1 μm filter
농도
~0.45 M in acetonitrile
기술
DNA synthesis: suitable
불순물
≤0.003% water
bp
84 °C (lit.)
mp
156-158 °C (lit.)
density
0.798 g/mL at 20 °C
SMILES string
c1nnn[nH]1
InChI
1S/CH2N4/c1-2-4-5-3-1/h1H,(H,2,3,4,5)
InChI key
KJUGUADJHNHALS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The product is ~0.45M solution of tetrazole in acetonitrile. Tetrazole ring plays significant role in various synthetic and industrial processes.
애플리케이션
Tetrazole solution is useful for DNA synthesis. It may be employed as a catalyst for the in situ synthesis of deoxyribonucleoside phosphoramidites. It may be used in the preparation of following anionic nucleotide-lipids:
- thymidine 3′-(1,2-dilauroyl-sn-glycero-3-phosphate) , diC12-3′-dT
- thymidine 3′-(1,2-dimyristoyl-sn-glycero-3-phosphate) diC14- 3′-dT
- thymidine-3′-(1,2-dipalmitoyl-sn-glycero-3-phosphate) diC16- 3′-dT
주의사항
Saturated solution at room temperature; storage below room temperature causes precipitation.
기타 정보
Catalyst used in the phosphite triester method of oligonucleotide synthesis for the coupling of (dialkylamino)phosphines
관련 제품
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
41.0 °F - closed cup
Flash Point (°C)
5 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Azidoazomethine-tetrazole isomerism in solution: A thermochemical study.
Cubero E, et al.
The Journal of Organic Chemistry, 63(7), 2354-2356 (1998)
A D Barone et al.
Nucleic acids research, 12(10), 4051-4061 (1984-05-25)
Deoxynucleoside phosphoramidites can be prepared in good yield from deoxynucleosides, bis- dialkylaminophosphines , and the corresponding dialkylamine hydrotetrazolide or tetrazole as catalysts. These phosphoramidites generated in situ lead to the direct synthesis of deoxyoligonucleotides on polymer supports.
Salim Khiati et al.
Bioconjugate chemistry, 20(9), 1765-1772 (2009-08-29)
A family of new anionic nucleotide based lipids featuring thymidine-3'-monophosphate as nucleotide and 1,2-diacyl-sn-glycerol as lipid moiety for in vitro delivery of nucleic acids is described. The nucleotide lipids were prepared in three steps starting from 1,2-diacyl-sn-glycerols and 2'-deoxythymidine-3'-phosphoramidite. Gel
J Nielsen et al.
Nucleic acids research, 14(18), 7391-7403 (1986-09-25)
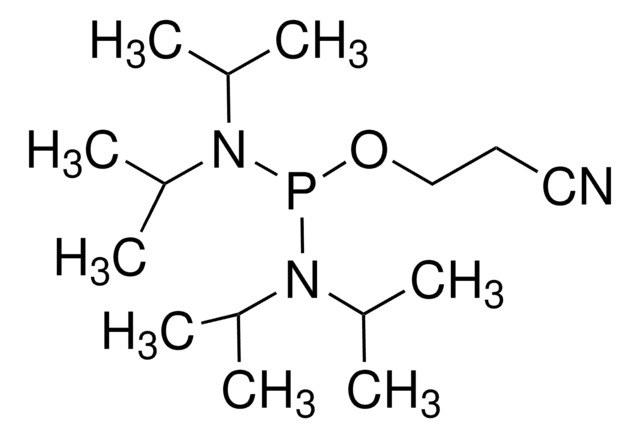
Deoxyribonucleoside phosphoramidites are prepared in situ from 5'-O,N-protected deoxyribonucleosides and 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite with tetrazole as catalyst, and the solutions applied directly on an automatic solid-phase DNA synthesizer. Using LCAA-CPG support and a cycle time of 12.5 min, oligonucleotides of 16-25
Adina Morozan et al.
ChemSusChem, 5(4), 647-651 (2012-03-06)
High-performance oxygen reduction reaction (ORR) catalysts based on metal-free nitrogen-containing precursors and carbon nanotubes are reported. The investigated systems allow the evaluation of the effect of nitrogen-containing groups towards ORR and the results show that the catalysts are compatible with
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.