추천 제품
분석
96%
형태
solid
광학 활성
[α]20/D -9.0°, c = 1 in methanol
mp
253-258 °C
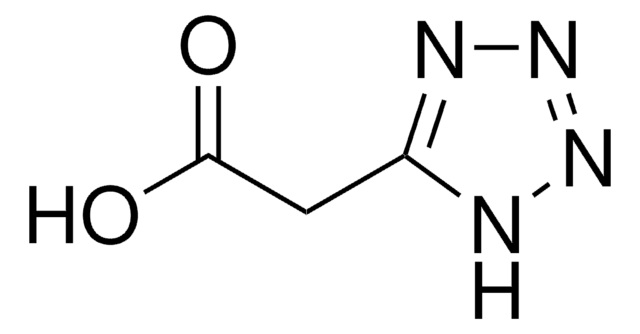
SMILES string
C1CN[C@@H](C1)c2nnn[nH]2
InChI
1S/C5H9N5/c1-2-4(6-3-1)5-7-9-10-8-5/h4,6H,1-3H2,(H,7,8,9,10)/t4-/m0/s1
InChI key
XUHYQIQIENDJER-BYPYZUCNSA-N
애플리케이션
(S)-(−)-5-(2-Pyrrolidinyl)-1H-tetrazole can be used as an organocatalyst:
- To prepare enantioselective chiral 1,2-oxazines from achiral ketones via an intramolecular Wittig reaction.
- To synthesize diastereoselective Michael addition products by addition of aliphatic aldehydes to β-nitrostyrene.
- In the direct asymmetric α-fluorination of linear and branched aldehydes using N-fluorobenzenesulfonamide as the fluorinating agent.
Organocatalyst used for:
- Direct asymmetric aldol reactions between acetone and aldehydes yielding β -hydroxy ketone and for synthesizing 1,1,1-trichloro-2-alkanols
- Mannich reactions for synthesis fo α -amino acids and generation of 1,4-diamines
- Conjugate additions of malonates to enones
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
가장 최신 버전 중 하나를 선택하세요:
Oelke, A. et al.
Synlett, 2548-2548 (2006)
Sirirat Kumarn et al.
Chemical communications (Cambridge, England), (30), 3211-3213 (2006-10-10)
A sequential, organocatalysed asymmetric reaction to access chiral 1,2-oxazines from achiral ketone starting materials is reported, which proceeds in moderate to good yields and excellent enantioselectivity.
Direct asymmetric α-fluorination of aldehydes
Steiner DD, et al.
Angewandte Chemie (International Edition in English), 117(24), 3772-3776 (2005)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.