추천 제품
분석
≥90.0% (GC)
양식
liquid
광학 활성
[α]/D -20±3°, c = 1 in ethanol
저장 온도
2-8°C
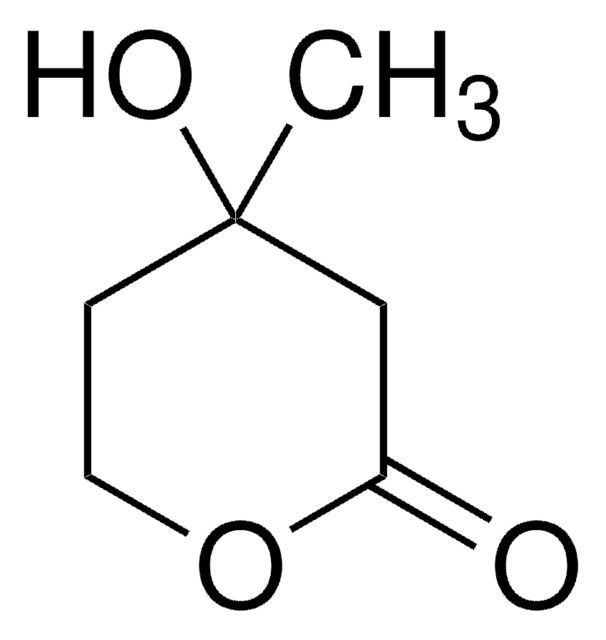
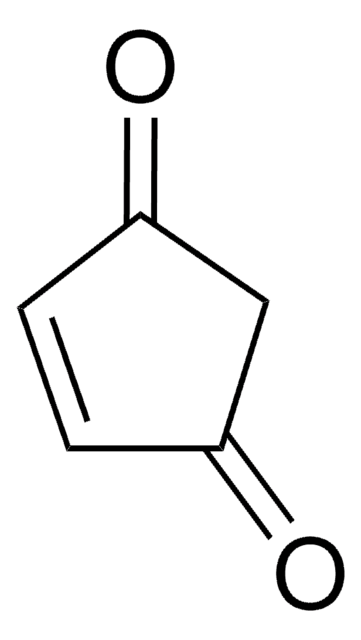
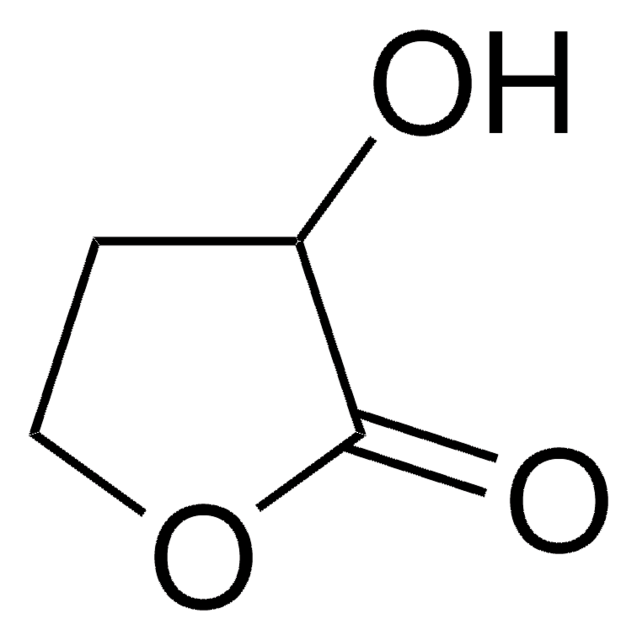
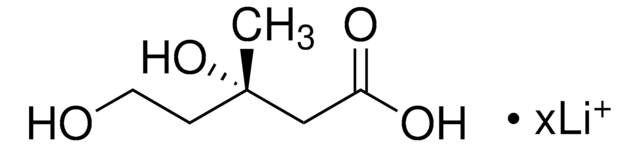
SMILES string
C[C@@]1(O)CCOC(=O)C1
InChI
1S/C6H10O3/c1-6(8)2-3-9-5(7)4-6/h8H,2-4H2,1H3/t6-/m1/s1
InChI key
JYVXNLLUYHCIIH-ZCFIWIBFSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
생화학적/생리학적 작용
Classical enantiomerically pure metabolite in biosynthetic pathways leading to sterols, terpenes, carotenoids, and other natural products.
포장
Amber colored round bottle
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Akira Honda et al.
Journal of lipid research, 48(5), 1212-1220 (2007-02-03)
We have developed a new sensitive and specific nonradioisotope assay method to measure the activity of HMG-CoA reductase, the rate-controlling enzyme in the cholesterol biosynthetic pathway. This method was based upon a stable isotope dilution technique by liquid chromatography-tandem mass
Jenna Waldron et al.
Annals of clinical biochemistry, 48(Pt 3), 223-232 (2011-03-01)
Mevalonic acid (MVA) is synthesized at an early and rate-limiting step in the biosynthesis of cholesterol by the enzyme hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase, and is a useful measure of statin efficacy or treatment. A liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Synthesis and HMG-CoA reductase inhibition of 2-cyclopropyl-4-thiophenyl-quinoline mevalonolactones.
Shikui Zhao et al.
Bioorganic & medicinal chemistry, 17(23), 7915-7923 (2009-11-03)
A novel series of 2-cyclopropyl-4-thiophenyl quinoline-based mevalonolactones were synthesized from the substituted anilines by several reactions. Among them, (4R,6S)-6-[(E)-2-(2-cyclopropyl-6-fluoro-4-(4-fluoro-thiophenyl)-quinoline-3-yl)-ethenyl]-3,4,5,6-tetrahydro-4-hydroxy-2H-pyran-2-one (1d), (4R,6S)-6-[(E)-2-(2-cyclopropyl-6-fluoro-4-(3-methoxy-thiophenyl)-quinoline-3-yl)-ethenyl]-3,4,5,6-tetrahydro-4-hydroxy-2H-pyran-2-one (1f) and (4R,6S)-6-[(E)-2-(2-cyclopropyl-6-fluoro-4,7-di(3-methoxy-thiophenyl)-quinoline-3-yl)-ethenyl]-3,4,5,6-tetrahydro-4-hydroxy-2H-pyran-2-one (1q) showed potent HMG-CoA reductase inhibitory activity comparable with pitavastatin.
Jessica Fuhrmeister et al.
Toxicology letters, 215(3), 219-227 (2012-10-25)
Statins are the most widely used drugs for the treatment of hypercholesterolemia. In spite of their overall favorable safety profile, they do possess serious myotoxic potential, whose molecular origin has remained equivocal. Here, we demonstrate in cultivated myoblasts and skeletal
Ya Sh Schwartz et al.
Bulletin of experimental biology and medicine, 148(3), 406-409 (2010-04-17)
We studied the effects of cholesterol, its oxidized derivatives mevalonate, and nuclear receptor agonists LXR, RXR, and FXR on the production of transforming growth factor-beta1 (TGF- beta1) by macrophages. After recruiting of macrophage monocytes into the focus of inflammation, the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.