455067
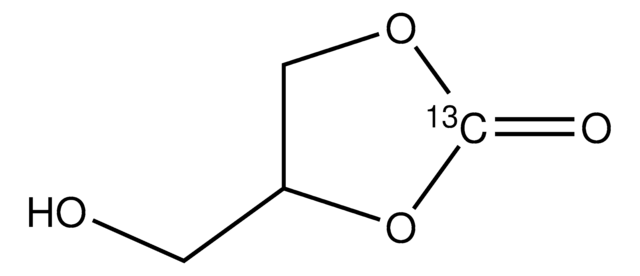
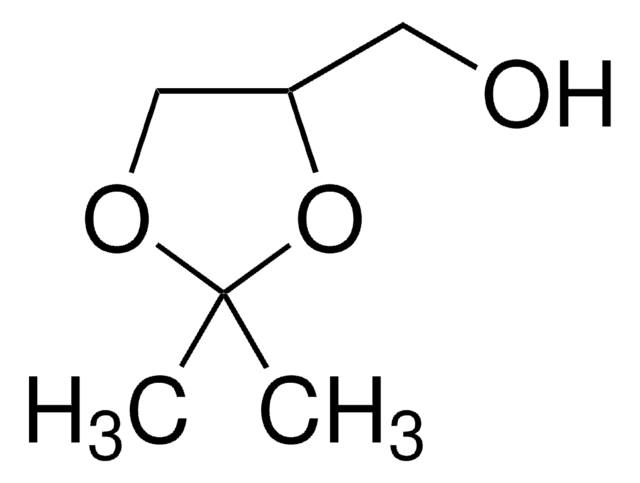
4-(Hydroxymethyl)-1,3-dioxolan-2-one
동의어(들):
(2-Oxo-1,3-dioxolan-4-yl)methanol, 3-Hydroxypropene carbonate, 3-Hydroxypropylene carbonate, 4-Methylolethylene carbonate, Glycerin carbonate, Glycerol 1,2-carbonate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C4H6O4
CAS Number:
Molecular Weight:
118.09
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
refractive index
n20/D 1.469 (lit.)
bp
137-140 °C/0.5 mmHg (lit.)
density
1.4 g/mL at 25 °C (lit.)
작용기
carbonate
hydroxyl
SMILES string
OCC1COC(=O)O1
InChI
1S/C4H6O4/c5-1-3-2-7-4(6)8-3/h3,5H,1-2H2
InChI key
JFMGYULNQJPJCY-UHFFFAOYSA-N
애플리케이션
4-(Hydroxymethyl)-1,3-dioxolan-2-one can be used as a building block to synthesize:
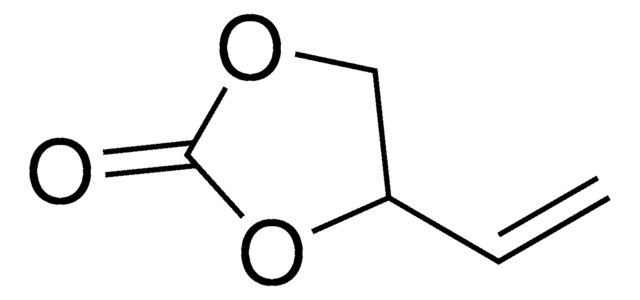
- 4-[(prop-2-en-1-yloxy)methyl]-1,3-dioxolan-2-one (AGC) via Williamson ether synthesis with 3-bromoprop-1-ene.
- Hyperbranched polyethers via copolymerization with cyclic carbonate containing phthalimide moieties.
- (2-Oxo-1,3-dioxolan-4-yl)methyl benzeneacetate by reacting with phenylacetyl chloride.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Polyurethanes with pendant hydroxyl groups: synthesis and characterization.
Ubaghs L, et al.
Macromolecular Rapid Communications, 25(3), 517-521 (2004)
Cyclic carbonates obtained by reactions of alkali metal carbonates with epihalohydrins.
Rokicki G and Kuran W.
Bulletin of the Chemical Society of Japan, 57(6), 1662-1666 (1984)
Converting wastes into added value products: from glycerol to glycerol carbonate, glycidol and epichlorohydrin using environmentally friendly synthetic routes.
Dibenedetto A, et al.
Tetrahedron, 67(6), 1308-1313 (2011)
Anuja Bhalkikar et al.
Journal of separation science, 39(23), 4484-4491 (2016-11-03)
An ion-moderated partition high-performance liquid chromatography method was developed for the separation and identification of common organic carbonates. The separation of organic carbonates was achieved on an ion exclusion column with an exchangeable hydrogen ion. An isocratic, aqueous mobile phase
Mario Pagliaro et al.
Angewandte Chemie (International ed. in English), 46(24), 4434-4440 (2007-05-02)
Today, industrial plants that produce glycerol are closing down and others are opening that use glycerol as a raw material, owing to the large surplus of glycerol formed as a by-product during the production of biodiesel. Research efforts to find
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.