Y0001480
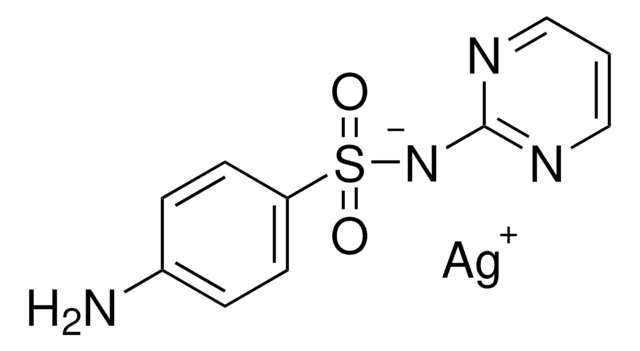
Sulfadiazine for identification of impurity F
European Pharmacopoeia (EP) Reference Standard
동의어(들):
Sulfadiazine, 4-Amino-N-(2-pyrimidinyl)benzenesulfonamide, N1-(Pyrimidin-2-yl)sulfanilamide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C10H10N4O2S
CAS Number:
Molecular Weight:
250.28
Beilstein:
6733588
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
sulfadiazine
제조업체/상표
EDQM
mp
253 °C (dec.) (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
Nc1ccc(cc1)S(=O)(=O)Nc2ncccn2
InChI
1S/C10H10N4O2S/c11-8-2-4-9(5-3-8)17(15,16)14-10-12-6-1-7-13-10/h1-7H,11H2,(H,12,13,14)
InChI key
SEEPANYCNGTZFQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Sulfadiazine for identification of impurity F EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
가장 최신 버전 중 하나를 선택하세요:
K Becker et al.
Medicine, 75(4), 185-194 (1996-07-01)
We performed a computerized search on sulfadiazine-associated nephrotoxicity reported in human immunodefiency virus (HIV)-infected patients in the international literature. Including an original case report, we summarized 35 acquired immunodefiency syndrome (AIDS) patients from 1987 to 1995 in an analysis comparing
U Kronawitter et al.
Deutsche medizinische Wochenschrift (1946), 118(46), 1683-1686 (1993-11-19)
A 45-year-old man with AIDS was treated for a recurrence of cerebral toxoplasmosis with sulphadiazine, 4 g, and pyrimethamine, 75 mg, daily. Owing to a lack of appetite and dysphagia he drank rather little water during the first week of
Charlotte Catalano-Pons et al.
Pediatric nephrology (Berlin, Germany), 19(8), 928-931 (2004-06-19)
Sulfadiazine-associated urinary calculi have been described in HIV-positive adult patients but rarely in children. We report two pediatric cases of sulfadiazine-induced nephrolithiasis and review 45 adult cases from the literature. One had a hyper-IgM syndrome and was treated with sulfadiazine
D I Simon et al.
Archives of internal medicine, 150(11), 2379-2384 (1990-11-01)
Toxoplasma gondii encephalitis is an important opportunistic infection in the acquired immunodeficiency syndrome, estimated to occur in 20,000 to 40,000 patients with acquired immunodeficiency syndrome in the United States by 1991. The combination of sulfadiazine and pyrimethamine is regarded as
S Hoffmann
Scandinavian journal of plastic and reconstructive surgery, 18(1), 119-126 (1984-01-01)
Topical antibacterial treatment is of major importance in the burn patient. Silver sulfadiazine is an effective agent with low toxicity and few side effects. Deposition of silver in tissues, and absorption of sulfadiazine are both minimal. Present and future problems
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.