PHR1343
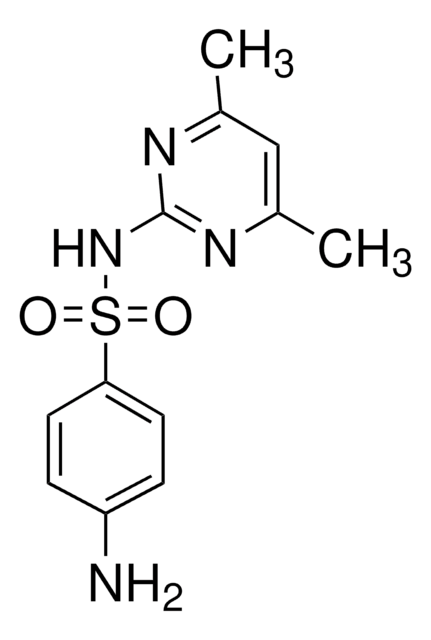
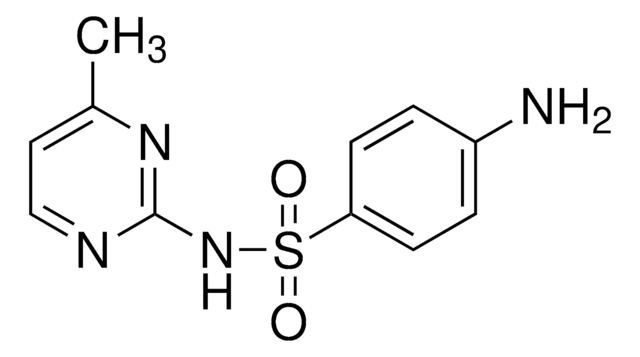
Sulfadiazine
Pharmaceutical Secondary Standard; Certified Reference Material
동의어(들):
Sulfadiazine, 4-Amino-N-(2-pyrimidinyl)benzenesulfonamide, N1-(Pyrimidin-2-yl)sulfanilamide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C10H10N4O2S
CAS Number:
Molecular Weight:
250.28
Beilstein:
6733588
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. S1800000
traceable to USP 1625009
API family
sulfadiazine
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
mp
253 °C (dec.) (lit.)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-30°C
SMILES string
Nc1ccc(cc1)S(=O)(=O)Nc2ncccn2
InChI
1S/C10H10N4O2S/c11-8-2-4-9(5-3-8)17(15,16)14-10-12-6-1-7-13-10/h1-7H,11H2,(H,12,13,14)
InChI key
SEEPANYCNGTZFQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Sulfadiazine is a bacteriostatic drug, that belongs to the class of medication referred as sulfonamide antibiotics. It is generally used in the treatment of cerebral meningitis, pneumonia, staphylococcal and streptococcal sepsis.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
애플리케이션
Sulfadiazine may be used as a pharmaceutical reference standard for the determination of the analyte in bulk drug and pharmaceutical formulations by high performance liquid chromatography.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
분석 메모
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
기타 정보
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
각주
To see an example of a Certificate of Analysis for this material enter LRAC3953 in the slot below. This is an example certificate only and may not be the lot that you receive.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Sulfadiazine
Pharmacopeia, US
United States Pharmacopeia/National Formulary, 4106-4106 (2018)
Sulfonamide antibiotics
Connor EE
Primary Care Update for OB/GYNS, 5(1), 32-35 (1998)
33 - Antimicrobial Drugs
Synthesis of Essential Drugs, 499-523 (2006)
Determination of the content and the related substances of silver sulfadiazine gel by HPLC
Li Y, et al.
Chinese Journal of Hospital Pharmacy, 24(8), 474-474 (2004)
Simultaneous determination of enrofloxacin, silver sulfadiazine, hydrocortisone acetate, hydrocortisone sodium succinate, and preservative excipients in pharmaceutical preparations using HPLC-DAD method
Rosa AM, et al.
Chromatographia, 80(11), 1641-1649 (2017)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.