추천 제품
Grade
pharmaceutical primary standard
API family
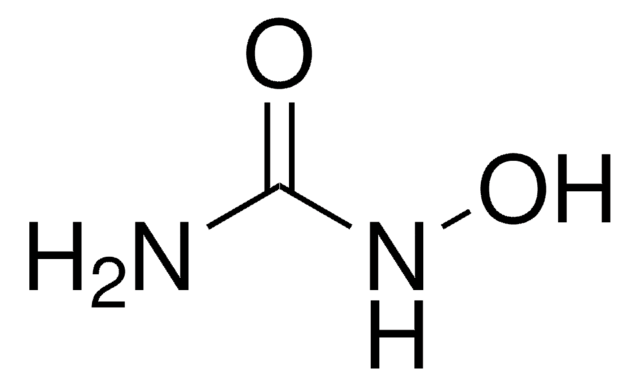
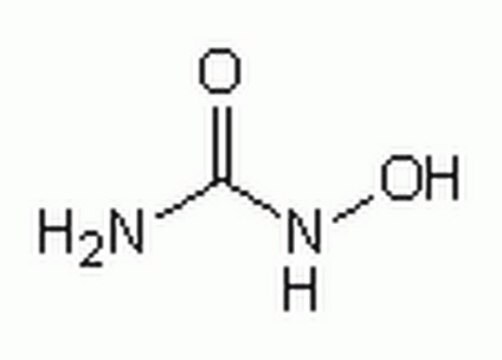
hydroxycarbamide
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
NC(=O)NO
InChI
1S/CH4N2O2/c2-1(4)3-5/h5H,(H3,2,3,4)
InChI key
VSNHCAURESNICA-UHFFFAOYSA-N
유전자 정보
human ... RRM1(6240) , RRM2(6241) , RRM2B(50484)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Hydroxycarbamide EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
Anti-neoplastic. Inactivates ribonucleoside reductase by forming a free radical nitroxide that binds a tyrosyl free radical in the active site of the enzyme. This blocks the synthesis of deoxynucleotides, which inhibits DNA synthesis and induces synchronization or cell death in S-phase.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Muta. 1B - Repr. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
A Tefferi et al.
Leukemia, 28(12), 2300-2303 (2014-05-06)
The impact of calreticulin (CALR) mutations on long-term survival in essential thrombocythemia (ET) was examined in 299 patients whose diagnosis predated 2006. Mutational frequencies were 53% for Janus kinase 2 (JAK2), 32% for CALR and 3% for MPL; the remaining
Patrick T McGann et al.
Current opinion in hematology, 18(3), 158-165 (2011-03-05)
Sickle cell anemia (SCA) is a well characterized severe hematological disorder with substantial morbidity and early mortality. Hydroxyurea is a potent inducer of fetal hemoglobin, and evidence over the past 25 years has documented its laboratory and clinical efficacy for
Alessandro M Vannucchi et al.
The New England journal of medicine, 372(5), 426-435 (2015-01-30)
Ruxolitinib, a Janus kinase (JAK) 1 and 2 inhibitor, was shown to have a clinical benefit in patients with polycythemia vera in a phase 2 study. We conducted a phase 3 open-label study to evaluate the efficacy and safety of
John J Strouse et al.
Pediatrics, 122(6), 1332-1342 (2008-12-03)
Hydroxyurea is the only approved medication for the treatment of sickle cell disease in adults; there are no approved drugs for children. Our goal was to synthesize the published literature on the efficacy, effectiveness, and toxicity of hydroxyurea in children
John J Strouse et al.
Pediatric blood & cancer, 59(2), 365-371 (2012-04-21)
Hydroxyurea is the only approved medication in the United States for the treatment of sickle cell anemia (HbSS) and is widely used in children despite an indication limited to adults. We review the evidence of efficacy and safety in children
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.